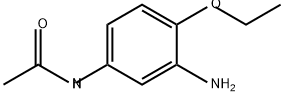
N-(3-amino-4-ethoxyphenyl)acetamide
- CAS No.
- 17026-81-2
- Chemical Name:
- N-(3-amino-4-ethoxyphenyl)acetamide
- Synonyms
- NCI-C01887;Ncgc00090849-01;AMINOETHOXYACETANILIDE;3'-AMINO-P-ACETOPHENETIDIDE;4'-Ethoxy-3'-aminoacetanilide;N-(3-amino-4-ethoxyphenyl)acetamide;1-Acetylamino-4-ethoxy-3-aminobenzene;Acetamide, N-(3-amino-4-ethoxyphenyl)-
- CBNumber:
- CB5908224
- Molecular Formula:
- C10H14N2O2
- Molecular Weight:
- 194.23
- MDL Number:
- MFCD00026773
- MOL File:
- 17026-81-2.mol
| Boiling point | 330.68°C (rough estimate) |
|---|---|
| Density | 1.1444 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| pka | 14.27±0.70(Predicted) |
| FDA UNII | 8W076B0J2D |
| EPA Substance Registry System | 3-Amino-4-ethoxyacetanilide (17026-81-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P |
N-(3-amino-4-ethoxyphenyl)acetamide price More Price(1)
N-(3-amino-4-ethoxyphenyl)acetamide Chemical Properties,Uses,Production
General Description
Brown powder.
Air & Water Reactions
N-(3-amino-4-ethoxyphenyl)acetamide may be sensitive to air and light . Insoluble in water.
Reactivity Profile
An amine and amide. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Fire Hazard
Flash point data for N-(3-amino-4-ethoxyphenyl)acetamide are not available; however, N-(3-amino-4-ethoxyphenyl)acetamide is probably combustible.
N-(3-amino-4-ethoxyphenyl)acetamide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| SynAsst Chemical. | 021-60343070 | China | 12740 | 55 | |
| Nanjing NingFan Pharm-Tech CO. LTD. | 17368696818 | China | 1984 | 58 | |
| Shaanxi Xihua Chemical Industry Co., Ltd | 17691182729 15529505138 | 1021@dideu.com | China | 9969 | 58 |
| Supplier | Advantage |
|---|---|
| Alchem Pharmtech,Inc. | 58 |
| SynAsst Chemical. | 55 |
| Nanjing NingFan Pharm-Tech CO. LTD. | 58 |
| Shaanxi Xihua Chemical Industry Co., Ltd | 58 |
17026-81-2(N-(3-amino-4-ethoxyphenyl)acetamide)Related Search:
1of3