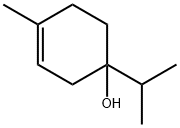
Terpinen-4-ol
- CAS No.
- 562-74-3
- Chemical Name:
- Terpinen-4-ol
- Synonyms
- 4-Terpineol;TERPINENE-4-OL;4-TERPINENOL;1-TERPINEN-4-OL;4-CARVOMENTHENOL;3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-;Terpin-4-ol;FEMA 2248;P-MENTH-1-EN-4-OL;terpineol-4
- CBNumber:
- CB6218216
- Molecular Formula:
- C10H18O
- Molecular Weight:
- 154.25
- MDL Number:
- MFCD00001562
- MOL File:
- 562-74-3.mol
- MSDS File:
- SDS
| Melting point | 137-188 °C |
|---|---|
| alpha | +25.2° |
| Boiling point | 88-90 °C |
| Density | 0.931 g/mL at 25 |
| FEMA | 2248 | 4-CARVOMENTHENOL |
| refractive index |
n |
| Flash point | 175 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| pka | 14.94±0.40(Predicted) |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Specific Gravity | 0.930.9265 (19℃) |
| Odor | at 100.00 %. pepper woody earth musty sweet |
| Odor Type | spicy |
| biological source | synthetic |
| optical activity | [α]20/D 27°, neat |
| Water Solubility | Very slightly soluble |
| Merck | 3935 |
| JECFA Number | 439 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | WRYLYDPHFGVWKC-UHFFFAOYSA-N |
| LogP | 2.99 |
| Substances Added to Food (formerly EAFUS) | 4-CARVOMENTHENOL |
| CAS DataBase Reference | 562-74-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | L65MV77ZG6 |
| NIST Chemistry Reference | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-(562-74-3) |
| EPA Substance Registry System | 4-Terpineol (562-74-3) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P264-P270-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36-37/39 |
| WGK Germany | 2 |
| RTECS | OT0175110 |
| HS Code | 29061990 |
| Hazardous Substances Data | 562-74-3(Hazardous Substances Data) |
Terpinen-4-ol price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W224820 | 4-Carvomenthenol natural, ≥95%, FG | 562-74-3 | sample-k | $62.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | W224820 | 4-Carvomenthenol natural, ≥95%, FG | 562-74-3 | 100g | $112 | 2024-03-01 | Buy |
| Sigma-Aldrich | W224820 | 4-Carvomenthenol natural, ≥95%, FG | 562-74-3 | 1kg | $514 | 2024-03-01 | Buy |
| Sigma-Aldrich | 03900590 | Terpinen 4-ol primary pharmaceutical reference standard | 562-74-3 | 50mg | $234 | 2024-03-01 | Buy |
| Sigma-Aldrich | W224847 | 4-Carvomenthenol ≥95%, FCC, FG | 562-74-3 | sample | $54.6 | 2024-03-01 | Buy |
Terpinen-4-ol Chemical Properties,Uses,Production
Overview
Essential oils and their components extracted from vegetable materials have been found to exhibit anti-microbial, anti-viral, anti-fungal, anti-oxidant, anti-inflammatory and anti-cancer activities[1–3].
Monoterpenes are major plant-derived secondary metabolites widely found in natural products, including fruits, vegetables and herbs and known to be associated with the plant defense mechanisms. The monoterpenes consist of two isoprene units, and are found in large amounts in essential oils[4,5]. In addition, many monoterpenes have been proposed to exert potent anticancer activity. Some of them reportedly displayed promising results in the prevention and treatment of different types of leukemia and cancers, such as breast, skin, pancreatic and colon cancers in rodents[6]. Notably, several of these compounds, among them Perillyl alcohol and limonene, are being testing in ongoing human studies[7–9].
Terpinen-4-ol, one of the primary active ingredients of the tea tree oil (TTO), consists of a mixture of more than 100 different compounds, and is found in a variety of aromatic plants (oranges, mandarins, origanum, New Zealand lemonwood tree, Japanese cedarand black pepper)[10].
Terpinen-4-ol is a potent bactericidal agent[11] that possesses antifungal properties[12]. Of particular interest is in vitro activity against Staphylococcus aureus and C. albicans[13,14]. It was shown that combining this natural substance and conventional drugs may help treat resistant yeast and bacterial infections.
Several recent reports have suggested that terpinen-4-ol induces antitumor effects by selectively causing necrotic cell death and cell-cycle arrest in melanoma cell lines, or by triggering caspase-dependent apoptosis in human melanoma cells, particularly in drug (Adriamycin) resistant cells[15,16]. Moreover, terpinen-4-ol was shown to elicit a dose-dependent cytotoxic response on human non-small cell lung cancer cells, presumably through the involvement of the mitochondrial apoptotic pathway[17].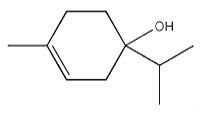
Figure 1 the chemical structure of Terpinen-4-ol
Biological activities
Terpinen-4-ol contained in TTO confers its various biological effects. Terpinen-4-ol is the major active component of tea tree oil. Terpinen-4-ol gained attention because of its antibacterial, antifungal, antiviral, and anti-inflammatory properties.
Antibacterial activity
erpinen-4-ol contained in TTO confers its remarkable antibacterial activity. Most bacteria are susceptible to TTO at concentrations of 1.0% or less; MICs in excess of 2% have been reported for organisms such as commensal skin staphylococci and micrococci, Enterococcus faecalis, and Pseudomonas aeruginosa[18]. TTO is for the most part bactericidal in nature, although it may be bacteriostatic at lower concentrations. The activity of TTO against antibiotic-resistant bacteria has attracted considerable interest, with methicillin-resistant Staphylococcus aureus (MRSA) receiving the most attention thus far. Since the potential to use TTO against MRSA was first hypothesized[19], several groups have evaluated the activity of TTO against MRSA, beginning with Carson et al.[20] who examined 64 MRSA isolates from Australia and the United Kingdom, including 33 mupirocin-resistant isolates. When the effects of terpinen-4-ol on S. aureus were examined, none was found to induce autolysis but was found to cause the leakage of 260-nmlightabsorbing material and to render cells susceptible to sodium chloride[21]. Electron microscopy of terpinen-4-ol-treated S. aureus cells revealed lesions similar to those seen after TTO treatment[22], including mesosome-like structures. In summary, the loss of intracellular material, inability to maintain homeostasis, and inhibition of respiration after treatment with terpinen-4-ol is consistent with a mechanism of action involving the loss of membrane integrity and function.
Antiprotozoal activity
Two publications show that TTO has antiprotozoal activity. TTO caused a 50% reduction in growth (compared to controls) of the protozoa Leishmania major and Trypanosoma brucei at concentrations of 403 mg/ml and 0.5 mg/ml, respectively[23]. In another study, TTO at 300 mg/ml killed all cells of Trichomonas vaginalis. There is also anecdotal in vivo evidence that TTO may be effective in treating Trichomonas vaginalis infections[24]. Further investigation showed that terpinen-4-ol contributed significantly to this activity.
Anti-Mites activity
It has been reported that lid scrub with different concentrations of TTO is effective in reducing Demodex mite counts and ocular surface inflammation associated with blepharitis, conjunctivitis, and keratitis[25, 26]. Terpinen-4-ol is the most active ingredient in TTO in exerting Demodex mite-killing effects.
Anti-inflammatory activity
Numerous recent studies now support the anecdotal evidence attributing anti-inflammatory activity to TTO. In vitro work over the last decade has demonstrated that TTO affects a range of immune responses, both in vitro and in vivo. It can inhibit the lipopolysaccharide-induced production of the inflammatory mediators tumor necrosis factor alpha (TNF-alpha), interleukin-1beta (IL-1beta), and IL-10 by human peripheral blood monocytes and that of prostaglandin E2. Terpinen-4-ol plays a major role in the anti-inflammatory effect of TTO. Terpinen-4-ol was able to diminish the production of TNF--alpha, IL-1 beta, IL-8, IL-10, and prostaglandin E2 by lipopolysaccharide-activated monocytes. Terpinen-4-ol also suppressed superoxide production by agonist-stimulated monocytes but not neutrophils[27]. TTO failed to suppress the adherence reaction of neutrophils induced by TNF-alpha stimulation or the casein-induced recruitment of neutrophils into the peritoneal cavities of mice. These studies identify specific mechanisms by which TTO may act in vivo to diminish the normal inflammatory response. In vivo, topically applied TTO has been shown to modulate the edema associated with the efferent phase of a contact hypersensitivity response in mice[28] but not the development of edema in the skin of nonsensitized mice or the edematous response to UVB exposure. This activity was attributed primarily to terpinen-4-ol. TTO and terpinen-4-ol applied after histamine injection reduced histamine-induced skin edema, and TTO also significantly reduced swelling induced by intradermal injection of compound 48/80[29]. Work has now shown that terpinen-4-ol modulates the vasodilation and plasma extravasation associated with histamine-induced inflammation in humans.
Anti-cancer
The anticancer effects of terpinen-4-ol are impressive in various types of cancer cells both in vitro and in vivo. Terpinen-4-ol is a major component of essential oil derived from several aromatic plants. It is used as an anti-inflammatory and antioxidant agent[15–17]. The contribution of terpinen-4-ol as an anti-cancer agent and the underlying signaling pathways of different types of cell death are unknown. Herein, it is shown that the mechanism of action of terpinen-4-ol is induction of apoptosis and not necrosis. It is also shown that terpinen-4-ol and various anticancer agents demonstrate a synergistic growth inhibitory effect by decreasing the survival of various cancer cell lines. Such combinations maybe expected to be more effective and less toxic since lower drug concentrations can be used for treating a wide range of cancers. Injection of terpinen-4-ol into the tumor remarkably inhibited tumor growth without any significant adverse effects. In search for more convenient routes of administration, two pharmaceutical formulations were prepared and tested for systemic administration, nano formulation and suspension. Nano formulations increased the surface area and therefore dramatically improved water solubility, bioavailability, effectiveness and efficiency. The suspension form was composed of small drops/molecules of the therapeutically active ingredient (the oil) in a suspension medium. Since the nanodrops were associated with serious toxicity (loss of body weight, mortality), the suspension approach that was devoid of any side effects was chosen for further exploration. The systemic administration of terpinen-4-ol by suspension was associated with a significant reduction in tumor size in the experimental nude mice.
References
- Martin KW, Ernst E (2004) Herbal medicines for treatment of fungal infections: a systematic review of controlled clinical trials. Mycoses 47: 87–92.
- Calcabrini A, Stringaro A, Toccacieli L, Meschini S, Marra M, Colone M, et al. (2004) Terpinen-4-ol, the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J Invest Dermatol 122: 349–360.
- Arweiler NB, Donos N, Netuschil L, Reich E, Sculean A (2000) Clinical and antibacterial effect of tea tree oil—a pilot study. Clin Oral Investig 4: 70–73.
- Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3: 408–414. PMID: 17576428
- Wagner KH, Elmadfa I (2003) Biological relevance of terpenoids. Overview focusing on mono-, diand tetraterpenes. Ann Nutr Metab 47: 95–106. PMID: 12743459
- Gould MN (1997) Cancer chemoprevention and therapy by monoterpenes. Environ Health Perspect 105 Suppl 4: 977–979. PMID: 9255590
- Da Fonseca CO, Masini M, Futuro D, Caetano R, Gattass CR, Quirico-Santos T (2006) Anaplastic oligodendroglioma responding favorably to intranasal delivery of perillyl alcohol: a case report and literature review. Surg Neurol 66: 611–615. PMID: 17145324
- da Fonseca CO, Schwartsmann G, Fischer J, Nagel J, Futuro D, Quirico-Santos T, et al. (2008) Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg Neurol 70: 259–266; discussion 266–257.
- Sobral Marianna Vieira X AL, Lima Tamires Cardoso, and de Sousa Damião Pergentino (2014) Antitumor Activity of Monoterpenes Found in Essential Oils. The Scientific World Journal 2014.
- Pino JA, Marbot R, Fuentes V (2003) Journal of Agricultural and Food Chemistry 51: 3836–3839.
- Loughlin R, Gilmore BF, McCarron PA, Tunney MM (2008). Lett Appl Microbiol 46: 428–433. doi: 10.1111/j.1472-765X.2008.02334.x
- Mondello F, De Bernardis F, Girolamo A, Cassone A, Salvatore G (2006) BMC Infect Dis 6: 158.
- Dryden MS, Dailly S, Crouch M (2004) J Hosp Infect 56: 283–286.
- Mertas A, Garbusinska A, Szliszka E, Jureczko A, Kowalska M, KrolW(2015) Biomed Res Int 2015: 590470. doi: 10.1155/2015/590470
- Greay SJ, Ireland DJ, Kissick HT, Levy A, Beilharz MW, Riley TV, et al. (2010) Cancer Chemother Pharmacol 65: 877–888.
- Calcabrini A, Stringaro A, Toccacieli L, Meschini S, Marra M, Colone M, et al. (2004) Terpinen-4-ol, The Main Component of Melaleuca Alternifolia (Tea Tree) Oil Inhibits the In Vitro Growth of Human Melanoma Cells. J Investig Dermatol 122: 349–360.
- Wu CS, Chen YJ, Chen JJ, Shieh JJ, Huang CH, Lin PS, et al. (2012) Terpinen-4-ol Induces Apoptosis in Human Nonsmall Cell Lung Cancer In Vitro and In Vivo. Evid Based Complement Alternat Med 2012: 818261. doi: 10.1155/2012/818261
- Banes-Marshall, L., P. Cawley, and C. A. Phillips. 2001. Br. J. Biomed. Sci. 58:139–145.
- Walsh, L. J., and J. Longstaff. 1987. The antimicrobial effects of an essential oil on selected oral pathogens. Periodontology 8:11–15.
- Carson, C. F., B. D. Cookson, H. D. Farrelly, and T. V. Riley. 1995. Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J. Antimicrob. Chemother. 35:421–424.
- Carson, C. F., B. J. Mee, and T. V. Riley. 2002. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 48:1914–1920.
- Reichling, J., A. Weseler, U. Landvatter, and R. Saller. 2002. Bioactive essential oils used in phytomedicine as antiinfective agents: Australian tea tree oil and manuka oil. Acta Phytotherapeutica 1:26–32.
- Mikus, J., M. Harkenthal, D. Steverding, and J. Reichling. 2000. In vitro effect of essential oils and isolated monoand sesquiterpenes on Leishmania major and Trypanosoma brucei. Planta Med. 66:366–368.
- Pen˜a, E. F. 1962. Melaleuca alternifolia oil—its use for trichomonal vaginitis and other vaginal infections. Obstet. Gynecol. 19:793–795.
- Gao YY, Di Pascuale M, Li W, et al. High prevalence of Demodex in eye lashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005; 46: 3094–3098.
- Liu J, Sheha H, Tseng SCG. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010; 10: 505–510.
- Brand, C., A. Ferrante, R. H. Prager, T. V. Riley, C. F. Carson, J. J. Finlay-Jones, and P. H. Hart. 2001. The water soluble-components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 50:213–219.
- Brand, C., M. A. Grimbaldeston, J. R. Gamble, J. Drew, J. J. Finlay-Jones, and P. H. Hart. 2002. Tea tree oil reduces the swelling associated with the efferent phase of a contact hypersensitivity response. Inflamm. Res. 51:236–244.
- Brand, C., S. L. Townley, J. J. Finlay-Jones, and P. H. Hart. 2002. Tea tree oil reduces histamine-induced oedema in murine ears. Inflamm. Res. 51: 283–289.
Chemical Properties
1-Terpinen-4-ol occurs as (+)-, (?)-, and racemic 1-terpinen-4-ol in many essential oils, for
example, from Pinus and Eucalyptus species, and in lavender oil. It is a colorless
liquid with a spicy, nutmeg-like, woody–earthy, and also lilac-like odor.
1-Terpinen-4-ol is a by-product in the synthesis of terpineol fromterpin hydrate
and occurs in commercial terpineol. Pure 1-terpinen-4-ol can be prepared from
terpinolene by photosensitized oxidation, reduction of the resulting 1-methyl-4-
isopropenyl-1-cyclohexene-4-hydroperoxide, and selective hydrogenation of the
corresponding alcohol.
It is used, for example, in artificial geranium and pepper oils and in perfumery for
creating herbaceous and lavender notes.
Chemical Properties
colourless or pale yellow liquid
Occurrence
4-Carvomenthenol (dextro) has been reported present in the oil of Cupressus macrocarpa lavender, Spanish origanum, Ledum palustre, Eucalyptus australiana var. A., Thuja occidentalis, etc. The l-form is present in the oil of Eucalyptus dives and in some other essences such as Xanthoxylum rhetsa, together with the racemic form. The racemic form is found in camphor oil. Reported found in fresh apple, apricots, orange juice, peel oils of orange, lemon, grapefruit, tangerines, anise, cinnamon, ginger and nutmeg.
Uses
Shows antioxidant effects. Antiseptic.
Uses
Reference Standard in the analysis of herbal medicinal products
Definition
ChEBI: A terpineol that is 1-menthene carrying a hydroxy substituent at position 4.
Taste threshold values
Taste characteristics at 30 ppm: sweet, citrus green with a tropical fruity character.
General Description
Produced and qualified by HWI pharma services GmbH.
Exact content by quantitative NMR can be found on the certificate.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Taste at 30 ppm
Anticancer Research
Also this molecule exhibits antitumor effects by apoptotic mechanism. Studies weredone in mice bearing A549 tumor xenografts (Quintans et al. 2013; Kiyan et al.2014).
Synthesis
One of several terpinenol isomers, depending on the position of the double bond and that of the hydroxyl group, this terpene, whose structure has been defined by Wallach, can be isolated by fractional distillation. It exists in nature as the dextro, levo and racemic isomer; the synthetic product is always optically inactive. The 1-terpineneol or 1-meththyl-4-isopropyl-3-cyclohexen-1-ol has been prepared by Wallach (Burdock, 1997).
Terpinen-4-ol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12813 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5870 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | mina@chuanghaibio.com | China | 18137 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3005 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
View Lastest Price from Terpinen-4-ol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-21 | Terpinen-4-ol
562-74-3
|
US $0.00 / KG | 1KG | 99% | 100 MT | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-04-15 | Terpinen-4-ol
562-74-3
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-04-15 | Terpinen-4-ol
562-74-3
|
US $50.00 / kg | 1kg | 99% | 5ton | Hebei Zhuanglai Chemical Trading Co.,Ltd |
-

- Terpinen-4-ol
562-74-3
- US $0.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Terpinen-4-ol
562-74-3
- US $0.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Terpinen-4-ol
562-74-3
- US $50.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd