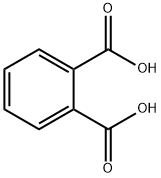
Phthalic acid
- CAS No.
- 88-99-3
- Chemical Name:
- Phthalic acid
- Synonyms
- 1,2-BENZENEDICARBOXYLIC ACID;O-PHTHALIC ACID;BENZENE-1,2-DICARBOXYLIC ACID;Phthalic acid,99%;Fluorescein EP Impurity B;NSC 5348;Phthalsure;Sunftal 20;THALIC ACID;phthalicaci
- CBNumber:
- CB6289456
- Molecular Formula:
- C8H6O4
- Molecular Weight:
- 166.13
- MDL Number:
- MFCD00002467
- MOL File:
- 88-99-3.mol
- MSDS File:
- SDS
| Melting point | 210-211 °C (dec.) (lit.) |
|---|---|
| Boiling point | 214.32°C (rough estimate) |
| Density | 1.59 g/cm3 at 15 °C |
| vapor pressure | 7.8 hPa (191 °C) |
| refractive index | 1.5100 (estimate) |
| Flash point | 168 °C |
| storage temp. | Store below +30°C. |
| solubility | methanol: 0.1 g/mL, clear |
| form | Powder |
| pka | 2.89(at 25℃) |
| color | White |
| PH | 3.2(1 mM solution);2.55(10 mM solution);2(100 mM solution); |
| Water Solubility | 7 g/L (25 ºC) |
| Merck | 14,7371 |
| BRN | 608199 |
| Dielectric constant | 5.1(Ambient) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | XNGIFLGASWRNHJ-UHFFFAOYSA-N |
| LogP | 0.73 at 20℃ |
| Indirect Additives used in Food Contact Substances | O-PHTHALIC ACID |
| FDA 21 CFR | 175.105 |
| CAS DataBase Reference | 88-99-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 6O7F7IX66E |
| NIST Chemistry Reference | 1,2-Benzenedicarboxylic acid(88-99-3) |
| EPA Substance Registry System | Phthalic acid (88-99-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-37/39 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TH9625000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29173980 | |||||||||
| Hazardous Substances Data | 88-99-3(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 7.9 g/kg (Shaffer) | |||||||||
| NFPA 704 |
|
Phthalic acid price More Price(43)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22298 | Phthalic acid for synthesis | 88-99-3 | 100g | $44.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22298 | Phthalic acid for synthesis | 88-99-3 | 1kg | $175 | 2024-03-01 | Buy |
| Sigma-Aldrich | 402915 | Phthalic acid ACS reagent, ≥99.5% | 88-99-3 | 1kg | $108 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1535899 | Phthalic acid United States Pharmacopeia (USP) Reference Standard | 88-99-3 | 50mg | $443 | 2024-03-01 | Buy |
| TCI Chemical | P0287 | Phthalic Acid >99.0%(GC)(T) | 88-99-3 | 25g | $21 | 2024-03-01 | Buy |
Phthalic acid Chemical Properties,Uses,Production
Description
Phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2. It is an isomer of isophthalic acid and terephthalic acid. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale.
Chemical Properties
PHTHALIC ACID,C6H4(COOH)2, mp 208 °C (ortho), 330 °C (meta and iso), the ortho form sublimes and the meta and iso forms decompose with heat, sp gr 1.593 (ortho). Phthalic acid is very slightly soluble in H2O, soluble in alcohol, and slightly soluble in ether. The solid form is colorless, crystalline
Uses
It is a dibasic acid, with pKa's of 2.89 and 5.51. The mono potassium salt, potassium hydrogen phthalate is a standard acid in analytical chemistry. Typically phthalate esters are prepared from the widely available phthalic anhydride. Reduction of phthalic acid with sodium amalgam in the presence of water gives the 1,3- cyclohexadiene derivative.
Uses
Phthalic Acid (Phenyl-13C6, D4) is labelled Phthalic Acid (P384480) which is an organic reagent used to synthesize phthalates.
Definition
ChEBI: A benzenedicarboxylic acid cosisting of two carboxy groups at ortho positions.
Production Methods
Phthalic acid is produced by the catalytic oxidation of naphthalene directly to phthalic anhydride and a subsequent hydrolysis of the anhydride.
Phthalic acid was first obtained by French chemist Auguste Laurent in 1836 by oxidizing naphthalene tetrachloride. Believing the resulting substance to be a naphthalene derivative, he named it "naphthalic acid". After the Swiss chemist Jean Charles Galissard de Marignac determined its correct formula, Laurent gave it its present name. Manufacturing methods in the nineteenth century included oxidation of naphthalene tetrachloride with nitric acid, or, better, oxidation of the hydrocarbon with fuming sulfuric acid, using mercury or mercury(II) sulfate as a catalyst.
Definition
phthalic acid: colourlesscrystalline dicarboxylic acid,C6H4(COOH)2; r.d. 1.6; m.p. 207°C.The two –COOH groups are substitutedon adjacent carbon atoms ofthe ring, the technical name beingbenzene-1,2-dicarboxylic acid. Theacid is made from phthalic anhydride(benzene-1,2-dicarboxylic anhydride,C8H4O3), which is made by the catalyticoxidation of naphthalene. Theanhydride is used in making plasticizersand polyester resins.
Synthesis Reference(s)
The Journal of Organic Chemistry, 30, p. 2414, 1965 DOI: 10.1021/jo01018a074
General Description
White crystals or fine white powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phthalic acid is a carboxylic acid. Phthalic acid is sensitive to exposure to extreme heat. Phthalic acid reacts violently with nitric acid. Phthalic acid is incompatible with sodium nitrite. Phthalic acid is also incompatible with oxidizers. .
Fire Hazard
Phthalic acid is combustible.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. A skin and mucous membrane irritant. Combustible when heated. In the form of dust (anhydride) it can explode. Mixtures with sodmm nitrite explode when heated. Violent reaction with HNO3. When heated to decomposition it emits acrid smoke and irritating fumes. Used in synthesis of dyes and dyestuffs, in medcines and perfumes.
Safety
The toxicity of phthalic acid is low with LD50 (mouse) of 550 mg/kg. However, many phthalate esters have been implicated as endocrine disruptors.
Purification Methods
Crystallise phthalic acid from water. [Beilstein 9 IV 3167.]
Isomers
Phthalic acid is one of three isomers of benzene dicarboxylic acid, the others being iso phthalic acid and terephthalic acid. Sometimes the term "phthalic acids" is used to refer to this family of isomers, but in the singular, "phthalic acid", refers exclusively to the ortho- isomer.
Phthalic acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
View Lastest Price from Phthalic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-18 | Phthalic Acid
88-99-3
|
US $120.00 / kg | 1kg | 99% | 10ton | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-11-18 | Phthalic acid
88-99-3
|
US $48.00 / g | 99.31% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-10-25 | Phthalic acid
88-99-3
|
US $1.00 / KG | 1KG | 99% | 10 mt | Hebei Weibang Biotechnology Co., Ltd |
-

- Phthalic Acid
88-99-3
- US $120.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Phthalic acid
88-99-3
- US $48.00 / g
- 99.31%
- TargetMol Chemicals Inc.
-

- Phthalic acid
88-99-3
- US $1.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd