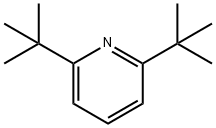
2,6-DI-TERT-BUTYLPYRIDINE
- CAS No.
- 585-48-8
- Chemical Name:
- 2,6-DI-TERT-BUTYLPYRIDINE
- Synonyms
- 2,6-DI-T-BUTYLPYRIDINE;2,6-Bis(tert-butyl)pyridine;NSC 175805;2,6-DI-TERT-BUTYLPYRIDINE;Pyridine, 2,6-di-tert-butyl-;2,6-Di-tert-butylpyridine>2,6-DI-TERT-BUTYLPYRIDINE 97%;2,6-Di-tert-butylpyridine ,96%;2,6-Di-tert-butylpyridine;2,6-DI-TERT-BUTYLPYRIDINE, >=97%
- CBNumber:
- CB6293437
- Molecular Formula:
- C13H21N
- Molecular Weight:
- 191.31
- MDL Number:
- MFCD00006306
- MOL File:
- 585-48-8.mol
- MSDS File:
- SDS
| Melting point | 2°C(lit.) |
|---|---|
| Boiling point | 100-101 °C/23 mmHg (lit.) |
| Density | 0.852 g/mL at 25 °C (lit.) |
| refractive index | 1.472-1.474 |
| Flash point | 72 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Liquid |
| pka | 3.58(at 25℃) |
| color | Clear yellow |
| Specific Gravity | 0.852 |
| Water Solubility | Miscible with alcohol, acetone, and hexane. Immiscible with water. |
| Merck | 14,3036 |
| BRN | 125886 |
| Dielectric constant | 3.3900000000000001 |
| Stability | Stable. Combustible. Incompatible with strong acids, strong oxidizing agents. |
| InChIKey | UWKQJZCTQGMHKD-UHFFFAOYSA-N |
| CAS DataBase Reference | 585-48-8(CAS DataBase Reference) |
| FDA UNII | OI9LF0H4MM |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 36/37/38-22 | |||||||||
| Safety Statements | 37/39-26-36/37/39-36 | |||||||||
| RIDADR | 3267 | |||||||||
| WGK Germany | 3 | |||||||||
| Hazard Note | Irritant | |||||||||
| HS Code | 29339900 | |||||||||
| NFPA 704 |
|
2,6-DI-TERT-BUTYLPYRIDINE price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 219584 | 2,6-Di-tert-butylpyridine ≥97% | 585-48-8 | 1g | $54.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 219584 | 2,6-Di-tert-butylpyridine ≥97% | 585-48-8 | 5g | $193 | 2024-03-01 | Buy |
| TCI Chemical | D1804 | 2,6-Di-tert-butylpyridine >97.0%(GC) | 585-48-8 | 5g | $142 | 2024-03-01 | Buy |
| TCI Chemical | D1804 | 2,6-Di-tert-butylpyridine >97.0%(GC) | 585-48-8 | 25g | $489 | 2024-03-01 | Buy |
| Alfa Aesar | H55761 | 2,6-Di-tert-butylpyridine, 97+% | 585-48-8 | 1g | $42.65 | 2024-03-01 | Buy |
2,6-DI-TERT-BUTYLPYRIDINE Chemical Properties,Uses,Production
Overview
2,6-Di-tert-butylpyridine is a weak base used in the preparation of 2, 6-di-tert-butylpyridine hydrotriflate. It is used as a proton scavenger to check the progress of the living polymerization of isobutylene. It is associated with cerric ammonium nitrate and used in the alfa-enolation of aldehydes. It is involved in the preparation of vinyl triflate using polymer-bound 2,6-di-tert-butylpyridine. Since it was first synthesized by Brown and Kanner[1], 2,6-di-tert-butylpyridine has attracted the interest of many researchers because of its unusually low basicity: with its two-alkyl substituents, DTBP is nevertheless a weaker base than unsubstituted pyridine in aqueous solution. Brown and Kanner[1] and others[2] proposed that the abnormally low basicity of DTBP was caused by steric hindrance to hydration of DTBPH+. Recent determinations of gas-phase proton affinities of DTBP and other alkyl-substituted pyridines showed that the basicity of DTBP in the gas phase was normal[2, 3], which confirmed that its weak basicity in water was due to solvent effects on DTBP and (or) DTBPH+. A complete analysis of the thermodynamic cycles linking the protonation processes of DTBP and other pyridines in the gas phase and in aqueous solution led Arnett and Chawla[2] to conclude that there was indeed some hindrance to the hydration of DTBPH+ as reflected in its abnormally low enthalpy of hydration. However, more recently Hopkins et al.[3], after investigating the protonation of additional tertbutylpyridines and repeating the thermodynamic determinations of Amett and Chawla[2] of DTBP, concluded from their new data that the hydration enthalpy of DTBPH+ was normal but that the corresponding entropy was abnormal; they suggested that the rotation of the water molecule attached to DTBPH+ and of -CMe3 was restricted. These results and conclusions were in agreement with the gas phase studies of Moet-Ner and Sieck[4] on the attachment of one water molecule to a series of pyridinium cations including DTBPH+.
Reference
- H. C. BROWN and B. KANNERJ. Am. Chem. Soc. 75, 3865 (1953).
- E. M. ARNETT and B. CHAWLAJ. Am. Chem. Soc. 101, 7141 (1979).
- H.P.HOPKINSD,.V.JAHAGIRDAP.RS,.MOULIKD,.H.AUE, H. M. WEBB,W. R. DAVIDSON and M. D. PEDLEY. J.. Am. Chem. Soc. 106,4341 (1984).
- M. MEOT-NEaRnd L. W. SIECK J. Am. Chem. Soc. 105, 2956 (1983)
Chemical Properties
dark brown liquid
Uses
2,6-Di-tert-butylpyridine was used as proton trapping agent to investigate the living polymerization of isobutylene. It was also used with cerric ammonium nitrate in the α-enolation of aldehydes leading to 1,4-dicarbonyl systems.
Uses
2,6-Di-tert-butylpyridine is used in the preparation of 2, 6-di-tert-butylpyridine hydrotriflate. It is used as a proton scavenger to check the progress of the living polymerization of isobutylene. It is associated with cerric ammonium nitrate and used in the alfa-enolation of aldehydes. It is involved in the preparation of vinyl triflate using polymer-bound 2,6-di-tert-butylpyridine.
Definition
ChEBI: 2,6-di-tert-butylpyridine is a member of pyridines.
General Description
Reactivity of 2,6-di-tert-butylpyridine with iron(III) tetraphenylporphyrin pi-cation radical has been examined by proton NMR spectroscopy. Reaction of 2,6-di-tert-butylpyridine with methyl iodide and methyl fluorosulfonate under high pressure has been reported.
Purification Methods
Redistil it from KOH pellets. [Beilstein 20 III/IV 2868.]
2,6-DI-TERT-BUTYLPYRIDINE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Suzhou Ryan Pharmachem Technology Co.,Ltd. | +86-512-68780025 +8618962125825 | sales@ryanchem.com | China | 136 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8808 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1176 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Zjartschem | +86-571 87238903 | jocelynpan@zjarts.com | CHINA | 988 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10311 | 58 |
| Accela ChemBio Inc. | +1-858-6993322 | info@accelachem.com | United States | 17777 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
View Lastest Price from 2,6-DI-TERT-BUTYLPYRIDINE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-06 | 2,6-DI-TERT-BUTYLPYRIDINE
585-48-8
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-08-14 | 2,6-DI-TERT-BUTYLPYRIDINE
585-48-8
|
US $0.00 / kg | 1kg | 99% | 50000KG/month | Hebei Mojin Biotechnology Co.,Ltd | |
 |
2023-05-09 | 2,6-DI-TERT-BUTYLPYRIDINE
585-48-8
|
US $0.00-0.00 / kg | 10kg | 98% | hundred kgs grade | Suzhou Ryan Pharmachem Technology Co.,Ltd. |
-

- 2,6-DI-TERT-BUTYLPYRIDINE
585-48-8
- US $10.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- 2,6-DI-TERT-BUTYLPYRIDINE
585-48-8
- US $0.00 / kg
- 99%
- Hebei Mojin Biotechnology Co.,Ltd
-

- 2,6-DI-TERT-BUTYLPYRIDINE
585-48-8
- US $0.00-0.00 / kg
- 98%
- Suzhou Ryan Pharmachem Technology Co.,Ltd.
585-48-8(2,6-DI-TERT-BUTYLPYRIDINE)Related Search:
1of3