ChemicalBook >> CAS DataBase List >>ISOVALEROPHENONE
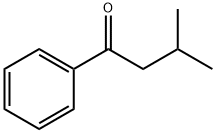
ISOVALEROPHENONE
- CAS No.
- 582-62-7
- Chemical Name:
- ISOVALEROPHENONE
- Synonyms
- 3-methyl-1-phenylbutan-1-one;3-methyl-1-phenyl-butan-1-one;1-Butanone, 3-methyl-1-phenyl-;ISOVALEROPHENONE;isobutanoylbenzene;Isovalerophenone>ISOVALEROPHENONE 99+%;ISOVALEROPHENONE 98+%;ISOVALEROPHENONE 98%;ISOBUTYL PHENYL KETONE
- CBNumber:
- CB6294287
- Molecular Formula:
- C11H14O
- Molecular Weight:
- 162.23
- MDL Number:
- MFCD00026486
- MOL File:
- 582-62-7.mol
- MSDS File:
- SDS
Last updated:2024-06-28 14:35:52
| Melting point | 225-235 °C |
|---|---|
| Boiling point | 72-74 °C3 mm Hg(lit.) |
| Density | 0.966 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | 99 °C |
| storage temp. | Sealed in dry,Room Temperature |
| form | clear liquid |
| color | Colorless to Light yellow to Light orange |
| BRN | 1861344 |
| LogP | 3.046 (est) |
| FDA UNII | U263XQR51P |
| EPA Substance Registry System | 1-Butanone, 3-methyl-1-phenyl- (582-62-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | DD8280000 | |||||||||
| HS Code | 2914390090 | |||||||||
| NFPA 704 |
|
ISOVALEROPHENONE price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S561150 | ISOVALEROPHENONE AldrichCPR | 582-62-7 | 250mg | $110 | 2024-03-01 | Buy |
| Sigma-Aldrich | 59920 | Isovalerophenone ≥98.0% (GC) | 582-62-7 | 10ml | $103.8 | 2024-03-01 | Buy |
| TCI Chemical | I0296 | Isovalerophenone >99.0%(GC) | 582-62-7 | 5mL | $75 | 2024-03-01 | Buy |
| TCI Chemical | I0296 | Isovalerophenone >99.0%(GC) | 582-62-7 | 25mL | $198 | 2024-03-01 | Buy |
| AK Scientific | 8629AB | Isovalerophenone | 582-62-7 | 5ml | $132 | 2021-12-16 | Buy |
ISOVALEROPHENONE Chemical Properties,Uses,Production
Chemical Properties
Colorless to pale yellow liquid. Insoluble in water, soluble in alcohol and
oils.
Fresh-fruity, sweet and moderately tenacious odor. The fruity notes are fresher, less
floral than those of the n-Valerophenone.
Production Methods
Isovalerophenone can be produced 1) from iso-Butyl phenylcarbinol by reduction. 2) from Benzonitrile plus iso-Butyl magnesium chloride, followed by hydration.
Definition
ChEBI: 3-Methyl-1-phenyl-1-butanone is an aromatic ketone.
ISOVALEROPHENONE Preparation Products And Raw materials
Raw materials
Preparation Products
ISOVALEROPHENONE Suppliers
Global( 55)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52849 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49979 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131957 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 94657 | 76 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 | market03@meryer.com | China | 40228 | 62 |
| TCI (Shanghai) Development Co., Ltd. | 021-67121386 | Sales-CN@TCIchemicals.com | China | 24529 | 81 |
| Energy Chemical | 021-021-58432009 400-005-6266 | sales8178@energy-chemical.com | China | 44689 | 61 |
| Shanghai Hanhong Scientific Co.,Ltd. | 021-54306202 13764082696 | info@hanhongsci.com | China | 42958 | 64 |
582-62-7(ISOVALEROPHENONE)Related Search:
1-Phenyl-3-methyl-1-butanone
3-methyl-1-phenyl-1-butanon
3-Methyl-1-phenyl-1-butanone
ISOVALEROPHENONE
ISOVALEROPHENONE 98%
ISOVALEROPHENONE 98+%
ISOVALEROPHENONE 99+%
isobutanoylbenzene
Isovalerophenone >=98.0% (GC)
ISOBUTYL PHENYL KETONE
1-Phenyl-3-methylbutan-1-one
Isovalerophenone>
3-methyl-1-phenyl-butan-1-one
3-methyl-1-phenylbutan-1-one
1-Butanone, 3-methyl-1-phenyl-
582-62-7
CH32CHCH2COC6H5
C6H5COCH2CHCH32
Organic Building Blocks
Ketones
C11 to C12
Carbonyl Compounds
Building Blocks
Building Blocks
C11 to C12
Carbonyl Compounds
Chemical Synthesis
Ketones
Organic Building Blocks