Boron carbide
- CAS No.
- 12069-32-8
- Chemical Name:
- Boron carbide
- Synonyms
- Norbide;B4-C;B4C HP;B4C HS;Tetrabor;B4C HD 20;B4C HD 15;B4C HD 07;BORON CARBIDE;Denkaboron 1200
- CBNumber:
- CB6315643
- Molecular Formula:
- CB4
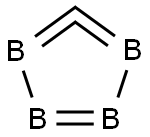
Lewis structure

- Molecular Weight:
- 55.25
- MDL Number:
- MFCD00011520
- MOL File:
- 12069-32-8.mol
- MSDS File:
- SDS
| Melting point | 2450°C |
|---|---|
| Boiling point | 3500°C |
| Density | 2.51 g/mL at 25 °C (lit.) |
| solubility | insoluble in H2O, acid solutions |
| form | powder |
| color | Black |
| Specific Gravity | 2.51 |
| Resistivity | 4500 (ρ/μΩ.cm) |
| Water Solubility | Insoluble in water. |
| Crystal Structure | Hexagonal |
| Merck | 14,1344 |
| Stability | Stable. Incompatible with oxidizing agents. Not flammable. |
| InChIKey | NOJMLSPGQSYAIT-UHFFFAOYSA-N |
| CAS DataBase Reference | 12069-32-8(CAS DataBase Reference) |
| FDA UNII | T5V24LJ508 |
| NIST Chemistry Reference | Boron carbide(12069-32-8) |
| EPA Substance Registry System | Boron carbide (B4C) (12069-32-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H332-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 20 | |||||||||
| Safety Statements | 22-36/37/39-38 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | ED7420000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28499010 | |||||||||
| NFPA 704 |
|
Boron carbide price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | GF92564916 | Boron carbide powder, 45 max. part. size (micron), weight 100?g, purity 99% | 12069-32-8 | 1EA | $460 | 2024-03-01 | Buy |
| Sigma-Aldrich | GF84490847 | Boron carbide granule, 5?mm nominal granule size, weight 100?g | 12069-32-8 | 1EA | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | GF75008156 | Boron carbide granule, 5?mm nominal granule size, weight 200?g | 12069-32-8 | 1EA | $195 | 2024-03-01 | Buy |
| Sigma-Aldrich | 378119 | Boron carbide powder, <10 μm, 98% | 12069-32-8 | 50g | $103 | 2024-03-01 | Buy |
| Sigma-Aldrich | 378100 | Boron carbide powder, 200 mesh, 98% | 12069-32-8 | 100g | $77.5 | 2024-03-01 | Buy |
Boron carbide Chemical Properties,Uses,Production
Physical and chemical properties
Boron carbide is black crystal with metallic luster, hardness ranks only second to diamond, higher than silicon carbide, Mohs hardness is 9.3. Chemical property is stable, it does not react with the acid solution, the formula is B4C, relative density is 2.52, melting point is 2350℃, boiling point is higher than 3500℃. The melt boron carbide can dissolve in a large amount of boron carbide graphitic carbon. Boron carbide is stable in dilute acid solution, the mixed acid of sulfuric acid and hydrofluoric acid, the mixed acid of sulfuric acid and nitric acid can decompose boron carbide. When heated to 1000℃, it is slowly oxidized to carbon dioxide and boron oxide in oxygen. Boron carbide has high thermal neutron capture capability, it is wear-resisting, it has semiconducting properties. In most cases, because boron carbide (of B4C) is used as control materials, it can meet the requirements of high-temperature reactors. Increasing the concentration of B10 in the boron carbide can improve the control efficiency of boron material. Boron carbide has a density of 2.51 × 103kg/m3, melting point is 2450 ℃, the thermal expansion coefficient (20~800 ℃) is 4.5 × 10-6/℃.

Figure 1 is the molecular structure of boron carbide.
Industrial boron carbide is mainly produced by methods of melting boron oxide in an electric arc furnace, and reacts with carbon can generat boron carbide. It can also obtained by carbon magnesium reduction method or hydrocarbon reduction method. Boron carbide is mainly used as abrasive, for grinding and polishing of industrial use.
Boron carbide is usually prepared by boric oxide and carbon in an electric furnace by high temperature heating. Reaction is as follows: 2B2O3 + 7C → B4C + 6CO.
Product quality general requires for grains with B4C is not less than 94%, milling class is not less than 90%.
Boron carbide is mainly used for grinding, milling, drilling and polishing of cemented carbide, precious stones and other hard materials. Moldings can be used for wear-resistant material, refractory material, used in the manufacture of hard resistant and corrosion-resistant ceramic water-resistant bearings, it is used as neutron control rods of nuclear reactor, it is also used for smelting boron steel, boron alloys and special welding. Alloy of boron carbide and aluminum (containing up to 50% B4C) is used for neutron shielding, reacting furnace screen cover and so on.
Boron carbide is packed in plastic bags, stored in a dry, clean warehouse.
More information is edited by Chemicalbook Xiaonan (2016-12-03).
Boron carbide ceramics
Boron carbide ceramics is a class of ceramics which the main chemical ingredient is boron carbide. The chemical formula of boron carbide is B4C, it belongs to hexagonal diamond crystal, there are 12 boron atoms in the unit cell, lattice parameter co = 1.212nm, ao = 0. 56nm. Crystal structure exists atom which can accommodate up to 0.18nm diameter, so it can remain the lithium or helium atoms within the crystal structure. Synthesis of boron carbide powder is mainly used carbon thermal reduction method, except the direct reduction with boron anhydride, it can also obtained in the presence of carbon (C), boron anhydride is reduced by Mg, the reaction is: 2B2O3 + 6Mg + C → B4C + 6MgO, the reaction temperature is 1000~1200 ℃. This reaction is highly exothermic, the final product requires H2SO4 or HCl acid pickling, then be washed with hot water to obtain more pure and fine grain size (0.1~5μm), no C boron carbide powder. Boron carbide ceramic is main hot pressing, hot isostatic pressing and non-pressure sintering is also used. Hot press sintering temperature is 2000~2100 ℃, generally Mg, Al, Cr, Si, Ti or a metal such as Al2O3, MgO, etc. or an oxide glass is added to use as sintering aid. The melting point of boron carbide is 2450 ℃, the theoretical density is 2.519g/cm3, elastic modulus is 360~460GPa, the thermal expansion coefficient is 5.73 × 10-6/K (300~1970K), the thermal conductivity at 200 ℃ is 24W/( m • K), hardness is 4950kg/mm2, just below the diamond and cubic boron nitride, it has a large thermal neutron capture cross-section. It is used to process precious stones, ceramics, molds, tools and bearings. It is also used as nozzles, bulletproof materials and nuclear reactor neutron absorber.
Uses
Uses
(1) Powder is used as abrasive material, the molded article can be used for wear-resistant material, also used for nuclear reactor.
(2) It is used for grinding carbide, precious stones and other hard materials, grinding, drilling and polishing, metal boride manufacturing and smelting boron steel, boron alloys and special welding.
References
1.https://www.azonano.com/article.aspx?ArticleID=3328
2.http://www.dynacer.com/materials/boron-carbide/
3.http://www.sciencedirect.com/science/article/pii/S0969804305003118
4.https://en.wikipedia.org/wiki/Boron_carbide#Uses
5.https://www.chemicalbook.com/ProductChemicalPropertiesCB6315643.htm
6.https://baike.baidu.com/item/%E7%A2%B3%E5%8C%96%E7%A1%BC/2363107?fr=aladdin
Description
Sodium tetraborate decahydrate/borax (anhydrous) is a clear, colorless or pale yellow hygroscopic substance with a faint odor of detergent. It is stable and is incompatible with powdered metalsand slightly soluble in water. It is extensively used in the industrial manufacturing of metallurgical fluxes, fiberglass, ceramics, fertilizers, enamels, heat-resistant glass (e.g., Pyrex), and other chemicals. It decomposes on heating or on burning producing toxic fumes including sodium oxide, reacts with strong oxidants, and in fire gives off irritating or toxic fumes (or gases).
Chemical Properties
hard, black, shiny crystal(s), -325 mesh with 99.5% purity; rhomb; hardness 9.3 Mohs; less brittle than most ceramics; does not burn in oxygen flame; used as an abrasive; Knoop hardness ~27GPa; produced by reducing B2O3 with carbon at 1400°C–2300°C; used in crucible form as a container for molten salts except molten caustic and as a 99.5% pure sputtering target for producing semiconductor and wear-resistant films [KIR78] [HAW93] [MER06] [CER91]
Physical properties
Hard black shiny crystals, fourth hardest material known after diamond, cubic boron nitride, and boron oxide. Does not burn in an O flame if temperature is 2 maintained below 983°C. Maximum operating temperature 2000°C (inert, reducing) or 600°C (oxidizing). Not attacked by hot HF or chromic acid. Used as abrasive, crucible container for molten salts except molten alkali hydroxides. In form of molded shape, used for pressure-blast nozzles, wire-drawing dies, and bearing surfaces for gauges. For grinding and lapping application available mesh sizes cover range 240 to 800.
Physical properties
Black hard crystal; density 2.50 g/cm3; hardness 9.3 Mohs; melts at 2,350°C; vaporizes above 3,500°C; insoluble in water and acid; inert to most chemicals at ordinary temperatures; rapidly attacked by hot alkalies.
Uses
Boron carbide (B4C) is a hard, black crystal that is used as an abrasive powder and as an additive to strengthen composite parts in aircraft.
Uses
Boron carbide is a hard boron-carbon ceramic material used in tank armor, bulletproof vests, engine sabotage powders, neutron absorber, cutting tools and dies and in brake linings of vehicles. It acts as an antioxidant additives in magnesia-carbon bricks. It is used as a precursor in the production of boron containing materials such as titanium boride.
Uses
Abrasive. In the manufacture of hard and chemicals-resistant ceramics or wear-resistant tools. Finely pulverized B4C can be molded under (considerable) pressure and heat. The resulting products are very wear-resistant, such as pressure-blast nozzle liners, thread guides, extrusion dies, and all types of extremely accurate plug, snap, and ring gages.
Application
Unlike natural minerals and rocks abrasives, boron carbide is a artificial electric-furnace abrasivescould uses in the encasement of spent nuclear waste, special optic fibers, high-gloss paints for the auto industry , and high-intensity electromagnets.
Definition
boron carbide: A black solid, B4C,soluble only in fused alkali; it is extremelyhard, over 9? on Mohs’scale; rhombohedral; r.d. 2.52; m.p.2350°C; b.p. >3500°C. Boron carbideis manufactured by the reduction ofboric oxide with petroleum coke inan electric furnace. It is used largelyas an abrasive, but objects can alsobe fabricated using high-temperaturepowder metallurgy. Boron nitride isalso used as a neutron absorber becauseof its high proportion ofboron–10.
Preparation
Boron carbide is prepared by reduction of boric oxide either with carbon or with magnesium in presence of carbon in an electric furnace at a temperature above 1,400°C. When magnesium is used, the reaction may be carried out in a graphite furnace and the magnesium byproducts are removed by treatment with acid.
Origin
Boron carbide is an artificial abrasive introduced in 1934 by the Norton Company under the name "Norbide." Washington Mills was the only producer of boron carbide in the United States in 2004.
Industrial uses
Boron carbide (B4C) is produced by the hightemperature(about 1371 to 2482°C) interactionof boric oxide, B2O3, and carbon in an electricalresistance-type furnace. It is a black, lustroussolid. It is used extensively as an abrasive,because its hardness approaches that of the diamond.It is also used as an alloying agent, particularlyin molybdenum steels.
Additionally, it is used in drawing dies andgauges, or into heat-resistant parts such as nozzles.The composition is either B6C or B4C; theformer is the harder but usually contains anexcess of graphite difficult to separate in thepowder. It can be used thus as a deoxidizingagent for casting copper, and also for lapping,since the graphite acts as a lubricant. Borofluxis B4C with flake graphite, used as a casting flux.B4C parts are fabricated by hot pressing,sintering, and sinter-HIPing (HIP = hot-isostaticpress). Industrially, densification is carriedout by hot pressing (2100 to 2200°C, 20to 40 MPa) in argon. The best properties areobtained when pure fine powder is densifiedwithout additives. Pressureless sintering to highdensity is possible using ultrafine powder, withadditives (notably carbon). Less expensive thanhot pressing, sintering also can be used for morecomplex shapes.
Special part formulations include bondingB4C with fused sodium silicate, borate frits,glasses, plastics, or rubbers to lend strength,hardness, or abrasion resistance. B4C-based cermets and MMC (especially Al/B4C, Mg/B4C, Ti/B4C), and CMCs (e.g., TiB2/B4C) haveunique properties, including superior ballisticperformance, that make these materials suitablefor highly specialized applications. Hightemperaturestrength, light weight, corrosionresistance, and hardness make these compositesespecially attractive. B4C shapes can bereaction-bonded using SiC as the bondingphase. B4C–C mixtures are formed, thenreacted with silicon to create the SiC bond. SiCalso can be used as a sintering aid for B4C, andvice versa.
Boron carbide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| PT CHEM GROUP LIMITED | peter68@ptchemgroup.com | China | 35425 | 58 | |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 984 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21639 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29886 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17365 | 58 |
Related articles
- Crystal Structure of Boron carbide
- Boron carbide (B?C) is a high-temperature semiconductor that can be used in new electronic applications.
- Jun 11,2024
- Boron Carbide: General Properties; Industrial Preparation; Industrial Applications and Uses
- The applications of boron carbide are as wear-resistant components. Armor tiles in military applications such as in light har....
- Jan 16,2024
View Lastest Price from Boron carbide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-08-21 | Boron carbide
12069-32-8
|
US $0.10 / KG | 1KG | 99.0% | 1000Tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2024-04-28 | Boron carbide | US $890.00 / T | 0.1T | 98% | 20T | Yujiang Chemical (Shandong) Co.,Ltd. | |
 |
2023-06-03 | Boron carbide
12069-32-8
|
US $100.00 / kg | 25kg | 99% | 500t/month | Henan Bao Enluo International TradeCo.,LTD |
-

- Boron carbide
12069-32-8
- US $0.10 / KG
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
-

- Boron carbide
- US $890.00 / T
- 98%
- Yujiang Chemical (Shandong) Co.,Ltd.
-

- Boron carbide
12069-32-8
- US $100.00 / kg
- 99%
- Henan Bao Enluo International TradeCo.,LTD