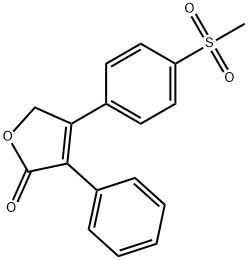
Rofecoxib
- CAS No.
- 162011-90-7
- Chemical Name:
- Rofecoxib
- Synonyms
- VIOXX;MK-0966;ROFECOXIB;Rofecoxid;AKOS 92137;Rofecoxib(Vioxx);Rofecoxib (MK0966);Rofecoxib (MK 966);Rofecoxib USP/EP/BP;Rofecoxib 162011-90-7
- CBNumber:
- CB6327130
- Molecular Formula:
- C17H14O4S
- Molecular Weight:
- 314.36
- MDL Number:
- MFCD00935806
- MOL File:
- 162011-90-7.mol
- MSDS File:
- SDS
| Melting point | 207°C |
|---|---|
| Boiling point | 577.6±50.0 °C(Predicted) |
| Density | 1.333±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble5mg/mL, clear (warmed) |
| form | powder |
| color | white to beige |
| Water Solubility | 9mg/L(25 ºC) |
| Merck | 14,8248 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 162011-90-7(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | rofecoxib; Vioxx |
| FDA UNII | 0QTW8Z7MCR |
| NCI Drug Dictionary | rofecoxib |
| ATC code | M01AH02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P501-P270-P264-P301+P312+P330 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LU5135000 | |||||||||
| HS Code | 2932.20.3000 | |||||||||
| NFPA 704 |
|
Rofecoxib price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0613 | Rofecoxib ≥98% (HPLC) | 162011-90-7 | 10mg | $89.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0613 | Rofecoxib ≥98% (HPLC) | 162011-90-7 | 50mg | $381 | 2024-03-01 | Buy |
| TCI Chemical | R0206 | Rofecoxib >98.0%(GC) | 162011-90-7 | 250mg | $60 | 2024-03-01 | Buy |
| TCI Chemical | R0206 | Rofecoxib >98.0%(GC) | 162011-90-7 | 1g | $162 | 2024-03-01 | Buy |
| Cayman Chemical | 10010260 | Rofecoxib ≥98% | 162011-90-7 | 50mg | $86 | 2024-03-01 | Buy |
Rofecoxib Chemical Properties,Uses,Production
Description
Rofecoxib ts a non-steroidal anti-inflammatory drug (NSAID) launched in Mexico, its first market, for the management of acute pain and the treatment of osteoarthritis (OA) and primary dysmenorrhea. Rofecoxib can be obtained by several different ways; one example is by arylation of a 4-bromofuranone with a phenylboronic acid under Suzuki conditions. Rofecoxib is a highly selective inhibitor of COX-2, the inducible isoform of cyclooxygenase and therefore exhibits a potent antiinflammatory activity without concomitant gastric or renal toxicities linked to the non-specific COX-1/2 inhibitors. In several clinical studies in patients with knee or hip osteoarthritis, Rofecoxib was evaluated at 12.5-50 mg doses once daily: it demonstrated efficacy for all primary and secondary endpoints at doses considerably weaker than those for classical non-specific NSAIDs, with good tolerance and less adverse effects. Selective COX-2 inhibitors potentially have a large spectrum of activity including new indications such as Alzheimer's disease, colorectal cancer, irritable bowel disease or urinary incontinence.
Chemical Properties
Off-White (Pale Yellow) Crystalline Powder
Originator
Merck (US)
Uses
Labeled Rizatriptan, intended for use as an internal standard for the quantification of Rizatriptan by GC- or LC-mass spectrometry.
Uses
antipsychotic
Uses
A selective cyclooxygenase-2 (COX-2) inhibitor. Use as an anti-inflammatory, analgesic.
Uses
Rofecoxib has been used in high performance bioaffinity chromatography.
Definition
ChEBI: A butenolide that is furan-2(5H)-one that is substituted by a phenyl group at position 3 and by a p-(methylsulfonyl)phenyl group at position 4. A selective cyclooxygenase 2 inhibitor, it was used from 1999 to 2004 for the tr atment of ostoarthritis, but was withdrawn following concerns about an associated increased risk of heart attack and stroke.
Indications
Rofecoxib is approved for the treatment of osteoarthritis, dysmenorrhea, and acute pain. The most common adverse reactions to rofecoxib are mild to moderate GI irritation (diarrhea, nausea, vomiting, dyspepsia, abdominal pain). Lower extremity edema and hypertension occur relatively frequently (about 3.5%). It is not metabolized by CYP2C9, so rofecoxib should not be subject to some of the interactions seen with celecoxib. However, its metabolism is increased by the coadministration of rifampin, which acts as a nonspecific inducer of hepatic metabolism.
brand name
Vioxx (Merck).
Biochem/physiol Actions
Rofecoxib is derived from furanone and has the ability to cross human placenta. Along with anti-inflammatory action, it possesses analgesic and antipyretic properties. Cytosolic hepatic enzymes are responsible for the metabolism of rofecoxib. It is known to cause oligohydramnios and ductus arteriosus constrictions. Rofecoxib inhibits the action of CYP1A2 (cytochrome P450 family 1 subfamily A member 2). It might be associated with aseptic meningitis. Rofecoxib is known to ameliorate the risk of colorectal adenoma, but might contribute to toxicity.
Mechanism of action
Rofecoxib is excreted primarily in the urine (72%) as metabolites. Less than 1% is excreted in the urine as unchanged drug, whereas approximately 14% is excreted in the feces as unchanged drug. Although the metabolism of rofecoxib has not been fully determined, the microsomal cytochrome P450 system appears to play only a minor role—a major difference in the metabolic routes of rofecoxib and celecoxib. The major metabolic route appears to form reduction of the dihydrofuranone ring system by cystolic enzymes to the to cis- and trans- dihydro derivatives. Also isolated is the glucuronide of a hydroxy derivative that results from CYP2C9 oxidative metabolism. None of the isolated metabolites of rofecoxib possess pharmacological activity as COX-1 or COX-2 inhibitors.
Pharmacokinetics
Rofecoxib has been synthesized by a number of synthetic routes that have been summarized elsewhere. It was the second selective COX-2 inhibitor to be marketed. Rofecoxib is well absorbed from the GI tract on oral administration, with peak plasma levels generally being attained within 2 to 3 hours of dosing. Bioavailability averages 93% following administration of a single dose. The area under the plasma concentration–time curve is increased in patients older than 65 years compared to younger adults and is increased slightly in black and Hispanic patients compared with white patients, but the difference is not considered to be clinically significant.
Clinical Use
Rofecoxib was indicated for the relief of the signs and symptoms of osteoarthritis, for the management of acute pain in adults, and for the treatment of primary dysmenorrhea.
Side effects
Rofecoxib causes a significantly lower incidence of upper-gastrointestinal adverse effects (perforations, ulcers, and bleeding) than conventional NSAIDs. Most common adverse events associated with rofecoxib are diarrhoea, headache, nausea, and upper respiratory tract infection.
Synthesis
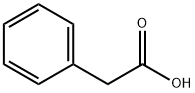
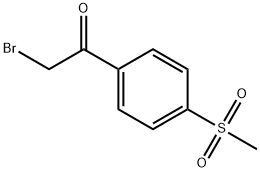
Rofecoxib can be obtained by different synthetic routes, e.g., by condensation of phenylacetic acid with ethyl bromoacetate to ethyl 2-phenylacetoxyacetate, which is then cyclized to a hydroxyfuranone. Subsequently, the hydroxyfuranone reacts with trifluoromethanesulfonic (triflic) anhydride to the corresponding triflate which reacts with LiBr to yield a bromofuranone. The bromofuranone is condensed with 4- (methylsulfanyl)phenylboronic acid to give 4-[4-(methylsulfanyl)phenyl]-3-phenylfuran- 2(5H)-one which is finally oxidized to rofecoxib.
IC 50
IC50 for COX-2: 1.8 × 10?8 M
IC50 for COX-1: 1.5 × 10?5 M
Dosage
Rofecoxib is indicated for relief of the signs and symptoms of osteoarthritis (recommended starting dose is 12.5 mg once daily, maximum recommended dose is 25 mg/d), for the management of acute pain in adults and for the treatment of primary dysmenorrhea (recommended initial doses are 50 mg once daily, use of rofecoxib for more than 5 d in management of pain has not been studied).
References
1) Chan et al. (1999), Rofecoxib [Vioxx, MK-0966; 4-(4′-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles; J. Pharmacol. Exp. Ther., 290 551 2) Catalla-Lawson et al. (2013), Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics and vasoactive eicosanoids; J. Pharmacol. Exp. Ther., 289 735
Rofecoxib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Ganmiao New material Technology Co., LTD | +86-17332992504 +86-17332992504 | sales8@hbganmiao.com | China | 299 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| Shenzhen Excellent Biotech Co., Ltd. | 13480692018 | ramyan@ex-biotech.com | CHINA | 954 | 58 |
View Lastest Price from Rofecoxib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Rofecoxib
162011-90-7
|
US $0.00 / G | 1G | 98%min | 300KG/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Rofecoxib
162011-90-7
|
US $38.00-50.00 / mg | 99.94% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Rofecoxib
162011-90-7
|
US $38.00-50.00 / mg | 99.94% | 10g | TargetMol Chemicals Inc. |