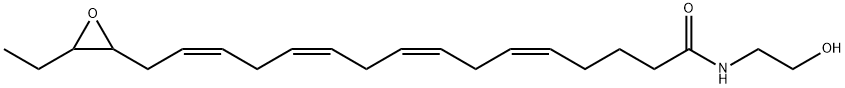
(±)17(18)-EpETE-Ethanolamide
- CAS No.
- 2123491-23-4
- Chemical Name:
- (±)17(18)-EpETE-Ethanolamide
- Synonyms
- (±)17(18)-EpETE-Ethanolamide;5,8,11,14-Hexadecatetraenamide, 16-(3-ethyl-2-oxiranyl)-N-(2-hydroxyethyl)-, (5Z,8Z,11Z,14Z)-
- CBNumber:
- CB66197340
- Molecular Formula:
- C22H35NO3
- Molecular Weight:
- 361.52
- MDL Number:
- MOL File:
- 2123491-23-4.mol
(±)17(18)-EpETE-Ethanolamide Chemical Properties,Uses,Production
Description
(±)17(18)-EpETE-Ethanolamide is an ω-3 endocannabinoid epoxide.1 It is formed from the endocannabinoid eicosapentaenoic ethanolamide (EPEA) via cytochrome P450 (CYP) epoxygenases and hydrolyzed by soluble epoxide hydrolase (sEH) and fatty acid amide hydrolase (FAAH). It is endogenously produced in LPS-stimulated BV-2 microglia cells and in BV-2 microglia cells supplemented with EPEA, an effect that can be reduced by the CYP inhibitor ketoconazole. (±)17(18)-EpETE-ethanolamide decreases IL-6 and nitrite production induced by LPS in BV-2 microglia and increases the production of IL-10. It inhibits platelet aggregation induced by arachidonic acid when used at a concentration of 50 μM but not aggregation induced by ADP , collagen, or ristocetin. It also induces relaxation of preconstricted bovine coronary arteries (ED50 = 1.1 μM) and inhibits VEGF-stimulated tubulogenesis in human microvascular endothelial cells (HMVECs). (±)17(18)-EpETE-ethanolamide is the predominant EPEA metabolite found in rat brain, and it has also been found in rat heart, kidney, spleen, and liver, as well as in pig brain. It activates cannabinoid receptor 1 (CB1) and CB2 with EC50 values of 18.5 and 1.4 nM, respectively, in a β-arrestin recruitment assay.
References
1. McDougle, D.R., Watson, J.E., Abdeen, A.A., et al. Anti-
(±)17(18)-EpETE-Ethanolamide Preparation Products And Raw materials
Raw materials
Preparation Products
(±)17(18)-EpETE-Ethanolamide Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Hongye Biotechnology Co. Ltd | 400-9205774 | sales@glpbio.cn | China | 6870 | 58 |
| ChemeGen(Shanghai) Biotechnology Co.,Ltd. | 18818260767 | sales@chemegen.com | China | 11289 | 58 |
| Shanghai Yifei Biotechnology Co. , Ltd. | 021-65675885 18964387627 | customer_service@efebio.com | China | 11975 | 58 |
| TargetMol Chemicals Inc. | 15002134094 | marketing@targetmol.cn | China | 18470 | 58 |