Isoamyl acetate
- CAS No.
- 123-92-2
- Chemical Name:
- Isoamyl acetate
- Synonyms
- 3-METHYLBUTYL ACETATE;PENTYL ACETATE;ISOPENTYL ACETATE;1-Butanol,3-methyl-,acetate;Banana oil;i-Amyl acetate;3-Methyl-1-butanol acetate;Isoamyl ethanoate;3-Methyl-1-butyl acetate;FEMA 2055
- CBNumber:
- CB7178555
- Molecular Formula:
- C7H14O2
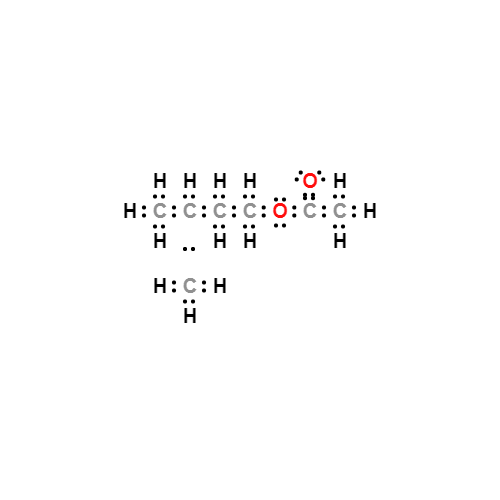
Lewis structure
- Molecular Weight:
- 130.18
- MDL Number:
- MFCD00008946
- MOL File:
- 123-92-2.mol
- MSDS File:
- SDS
| Melting point | -78 °C (lit.) |
|---|---|
| Boiling point | 142 °C/756 mmHg (lit.) |
| Density | 0.876 g/mL at 25 °C (lit.) |
| vapor density | 4.5 (vs air) |
| vapor pressure | 5 mm Hg ( 25 °C) |
| refractive index |
n |
| FEMA | 2055 | ISOAMYL ACETATE |
| Flash point | 77 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | ethanol: soluble1ml/3ml, clear, colorless (60%ethanol) |
| color | Clear Colorless |
| Odor | Banana-like odor |
| explosive limit | 1-10%(V) |
| Evaporation Rate | 0.42 |
| Odor Type | fruity |
| Water Solubility | 0.20 g/100 mL. Slightly soluble |
| Merck | 14,5111 |
| JECFA Number | 43 |
| BRN | 1744750 |
| Henry's Law Constant | 10.25 at 37 °C (static headspace-GC, Bylaite et al., 2004) |
| Exposure limits | TLV-TWA 100 ppm (~530 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 125 ppm (~655 mg/m3); IDLH 3000 ppm (NIOSH). |
| Dielectric constant | 5.6(20℃) |
| LogP | 2.7 at 35℃ |
| Substances Added to Food (formerly EAFUS) | ISOAMYL ACETATE |
| FDA 21 CFR | 172.515 |
| CAS DataBase Reference | 123-92-2(CAS DataBase Reference) |
| FDA UNII | Z135787824 |
| NIST Chemistry Reference | 1-Butanol, 3-methyl-, acetate(123-92-2) |
| EPA Substance Registry System | Isoamyl acetate (123-92-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226 | |||||||||
| Precautionary statements | P210 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 10-66-36/37/38-R66-R10 | |||||||||
| Safety Statements | 23-25-2-36/37/39-26-16-S25-S23-S2 | |||||||||
| RIDADR | UN 1104 3/PG 3 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 100 ppm (525 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | NS9800000 | |||||||||
| Autoignition Temperature | 680 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29153900 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rat > 5000 mg/kg | |||||||||
| IDLA | 1,000 ppm | |||||||||
| NFPA 704 |
|
Isoamyl acetate price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W205532 | Isoamyl Acetate natural, ≥97%, FCC, FG | 123-92-2 | 1kg | $81.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | W205532 | Isoamyl Acetate natural, ≥97%, FCC, FG | 123-92-2 | 4kg | $255 | 2024-03-01 | Buy |
| Sigma-Aldrich | W205532 | Isoamyl Acetate natural, ≥97%, FCC, FG | 123-92-2 | 9kg | $381 | 2024-03-01 | Buy |
| Sigma-Aldrich | W205532 | Isoamyl Acetate natural, ≥97%, FCC, FG | 123-92-2 | 20kg | $625 | 2024-03-01 | Buy |
| Sigma-Aldrich | W205508 | Isoamyl Acetate ≥95%, FCC, FG | 123-92-2 | 1kg | $76.5 | 2024-03-01 | Buy |
Isoamyl acetate Chemical Properties,Uses,Production
Chemical properties
Isoamyl acetate appears as colorless transparent liquid with pleasant banana aroma, being volatile. It is miscible with ethanol, ethyl ether, benzene, carbon disulfide and other organic solvents, being almost insoluble in water.
Uses
1. It is widely used to configure a variety of fruit flavors, such as Sydney, banana and some other types. There is also some appropriate application in the smoke and daily make-up essence is also appropriate application.
2. It can be used in the fragrance of heavy flowers such as chypre, sweet-scented osmanthus, hyacinth and heavy oriental flavor, being able to give the fragrance of fresh flower and adjust the fragrance odor with the dosage being often less than 1%. It is also applicable to the smiling flower type. It is also the major spices for the deployment of edible raw pear and banana flavor. It is also used in apple, pineapple, cocoa, cherry, grape, raspberry, strawberry, peach, caramel, cola, cream, coconut and vanilla bean. Also commonly used in wine and tobacco flavor.
3. Isoamyl acetate is a kind of edible fragrance allowed in China. It can be used to prepare fruit flavors such as strawberry, pineapple, bayberry, pear, apple, grape and banana. The dosage is 2700mg/kg in normal chewing gum. 190 mg/kg in confectionery; 120 mg/kg in pastry; 56 mg/kg in ice cream; 28 mg/kg in soft drinks.
4. Isoamyl acetate is an important solvent, being able to dissolve nitrocellulose, ester gum, vinyl resin, coumarone resin, rosin, frankincense, dama resin, mountain resin and castor oil. In Japan, 80% of the goods are used as spices, having a strong fruit flavor, like pears, bananas, apples and other fragrance. It is widely used in a variety of edible fruit flavor. In the smoke spice and daily cosmetic spice, there is also appropriate application. It is also used in rayon, dyes, artificial pearls, penicillin extraction and so on.
5. GB 2760~96 provides it as allowable food flavors. It can also be taken as a solvent, being the major raw material for the preparation of pear and banana flavor. It is commonly used in alcohol and tobacco flavor, also used in the preparation of apples, pineapple, cocoa, cherries, grapes, strawberries, peaches, butter, coconut and other fragrance preparation.
6. It can be used as chromatographic standard substance, extraction agent and solvent
7. It can be taken as solvent, for chromium determination, photography, printing and dyeing and the extraction agent of iron, cobalt and nickel.
Production method
Isoamyl acetate is obtained through the esterification between the acetate with the isoamyl alcohol separated from the fusel oil under the catalysis of sulfuric acid.
It is derived from the esterification between acetic acid and isoamyl alcohol. In the mixture of acetic acid and amyl alcohol, add sulfuric acid for esterification reaction, followed by neutralization with sodium carbonate (or caustic soda) with further calcium chloride dehydration to obtain the crude ester, and then refined by distillation to derive the finished products.
Isoamyl acetate is presented in bananas and cocoa beans and is commercially manufactured using pentanol separated from fusel oil as raw materials. Isoamyl alcohol and sulfuric acid have been added to glacial acetic acid for heating reflux reaction. When the temperature of the top of the column reached 132 °C, the esterification was complete. After cooling, wash with water, and neutralize with 10% NaOH solution and then washed with water to neutral, and finally dried with anhydrous calcium chloride after distillation, collecting 138~143 ° C fractions, being the product.
CH3COOH (CH3) 2CHCH2CH2OH [H2SO4] → CH3COOCH2CH2CH (CH3) 2 + H2O
Content analysis
This was determined by the method I in the ester assay (OT-18). The amount of sample taken was 800 mg. The equivalent factor (e) in the calculation is 65.10.
Alternatively, apply the non-polar column in the gas chromatography (GT-10-4) for measurement;
Toxicity
ADI 0 to 3.7 (FAO/WHO, 1994);
LD5016.6 g/kg (rat, oral);
Usage limit
FEMA (mg/kg): soft drinks 28; cold drinks 56; candy 190; baked goods 120; pudding 100; take appropriate amount as limit (FDA § 172.515, 2000);
Hazards & Safety Information
Category: Flammable liquids
Toxicity classification: Low toxicity
Acute Toxicity: Oral-Rat LD50: 16600 mg/kg
EXPLOSIVES HAZARDOUS CHARACTERISTICS: it is explosive if its vapors are blended with air
Flammability and Hazardous characteristics: it is easily flammable with burning releasing irritant smoke
Storage and transportation characteristics: Treasury: ventilated, low temperature and dry; store and transport it separately from oxidant.
Extinguishing agent: dry powder, carbon dioxide, foam, mist water
Occupational Standard: TWA 525 mg/m3; STEL 650 mg/m3
Description
In commercial practice amyl invariably means isoamyl, unless it is prefaced by the n- for normal. Isoamyl acetate has a powerful, fruity odor with a bittersweet taste reminiscent of pear. If impure, the odor is strong, penetrating, and almost shocking. Usually prepared by esterification of commercial isoamyl alcohol with acetic acid.
Chemical Properties
Isoamyl Acetate is a strongly fruity-smelling liquid and has been identified in many fruit aromas. It is the main component of banana aroma and is, therefore, also used in banana flavors.
All isomers of amyl acetate are highly flammable, colorless to yellow, watery liquids.
Isoamyl acetate has a fruity, banana, sweet, fragrant, powerful odor with a bittersweet taste reminiscent of pear. If impure, the odor is strong, penetrating and almost shocking. In commercial practice, amyl invariably means isoamyl.
Physical properties
Clear, colorless liquid with a banana or pear-like odor. Odor threshold concentration is 7 ppm (quoted, Keith and Walters, 1992). A detection odor threshold concentration of 18 μg/m3 (3.4 ppbv) was determined by Katz and Talbert (1930).
Occurrence
Reported to be found in a number of naturally occurring products, including apple, banana, cocoa bean, coffee, cognac, grape, peach, pear, pineapple and strawberry
Uses
Sting pheromone of the honeybee.1
Uses
Isoamyl acetate is used to impart pear flavorto mineral waters and syrups, in perfumes, inthe manufacture of artificial silk or leather, inphotographic films, in dyeing textiles, and asa solvent.
Uses
banana oil is a carrier oil. The banana family is of more interest for its nutritional value rather than for its botanical properties. The use of plantain juice as an antidote for snake bites has been reported in parts of Southeast Asia since 1916.
Uses
In alcohol solution as a pear flavor in mineral waters and syrups; as solvent for old oil colors, for tannins, nitrocellulose, lacquers, celluloid, and camphor; swelling bath sponges; covering unpleasant odors, perfuming shoe polish; manufacture of artificial silk, leather or pearls, photographic films, celluloid cements, waterproof varnishes, bronzing liquids, and metallic paints; dyeing and finishing textiles. A special grade of the amyl acetate has been used for burning in the Hefner lamp serving as a photometric standard.
Definition
ChEBI: The acetate ester of isoamylol.
Preparation
By the esterification of commercial isoamyl alcohol with acetic acid
Production Methods
The commerical-grade isoamyl acetate is prepared by the esterification of amyl alcohol (often fusel oil) with acetic acid and a small amount of sulfuric acid as the catalyst .
Taste threshold values
FEMA PADI: 24.491 mg
General Description
Oily liquid; colorless; banana odor. Floats and mixes with water. Flammable, irritating vapor is produced .
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Isoamyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Isoamyl acetate can react violently with oxidizing materials, nitrates, strong alkalis and strong acids.
Hazard
Flammable, moderate fire risk. Irritant. Explosive limits in air 1–7.5%.
Health Hazard
Isoamyl acetate exhibits low toxicity; thetoxic effects are comparable to those of n amyl acetate. The toxic symptoms includeirritation of the eyes, nose, and throat;fatigue; increased pulse rate; and narcosis.Inhalation of its vapors at 1000 ppm for30 minutes may cause irritation, fatigue, andrespiratory distress in humans. It is more narcotic than are the lower acetic esters. TheLD50 value in rabbits is on the order of7000 mg/kg.
Fire Hazard
FLAMMABLE. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area. When heated emits acrid fumes. When exposed to flames can react vigorously with reducing materials.
Flammability and Explosibility
Flammable
Potential Exposure
(n-isomer): Primary irritant (w/o allergic reaction), (sec-isomer) Human Data. Amyl acetates are used as industrial solvents and in the manufacturing and dry-cleaning industry; making artificial fruit-flavoring agents; cements, coated papers, lacquers; in medications as an inflammatory agent; pet repellents, insecticides and miticide. Many other uses.
Carcinogenicity
Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.
Source
Identified among 139 volatile compounds identified in cantaloupe (Cucumis melo var. reticulates cv. Sol Real) using an automated rapid headspace solid phase microextraction method (Beaulieu and Grimm, 2001).
Environmental Fate
Chemical/Physical. Slowly hydrolyzes in water forming 3-methyl-1-butanol and acetic acid.
Shipping
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.
Purification Methods
Dry the acetate with finely divided K2CO3 and fractionally distil it. [Beilstein 2 IV 157.]
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrates. May soften certain plastics.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Isoamyl acetate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Xiangduo Industry Co., Ltd. | +86-15981848961 +86-15981848961 | sales@xiangduochem.com | China | 497 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 | sales@czwytech.com | CHINA | 904 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
Related articles
- Isoamyl Acetate: Physical Properties, Industrial Applications and Enzymatic Synthesis
- Isoamyl acetate is a colorless liquid with a fruity aroma, with the molecular formula C7H14O2.
- Nov 6,2024
View Lastest Price from Isoamyl acetate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
|
|
2024-11-20 | Isoamyl acetate
123-92-2
|
US $1.00 / KG | 1KG | 99% | 10 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-11-14 | Isoamyl acetate
123-92-2
|
US $142.00 / KG | 1KG | 98% | 1-20mt | Baoji Guokang Bio-Technology Co., Ltd. | |
 |
2024-10-11 | Isoamyl acetate
123-92-2
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD |
-
- Isoamyl acetate
123-92-2
- US $1.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Isoamyl acetate
123-92-2
- US $142.00 / KG
- 98%
- Baoji Guokang Bio-Technology Co., Ltd.
-

- Isoamyl acetate
123-92-2
- US $5.00-2.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
123-92-2(Isoamyl acetate)Related Search:
1of4








