nitryl chloride
- CAS No.
- 13444-90-1
- Chemical Name:
- nitryl chloride
- Synonyms
- Nitro chloride;nitryl chloride;Nitroxyl chloride;Nitryl chloride ((NO2)Cl)
- CBNumber:
- CB72079351
- Molecular Formula:
- ClNO2
Lewis structure
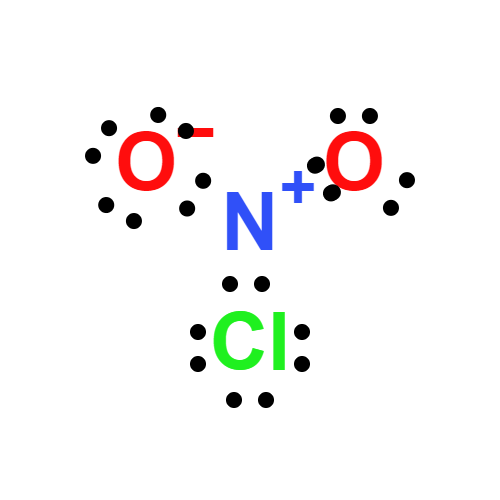
- Molecular Weight:
- 81.46
- MDL Number:
- MOL File:
- 13444-90-1.mol
| Melting point | -145° |
|---|---|
| Boiling point | bp -14.3° |
| Density | d0liq 1.37; d16liq 1.33 |
| vapor density | 2.81 g/L |
| form | colorless gas |
| color | Colorless gas darkens on storage |
| EWG's Food Scores | 1 |
| FDA UNII | UJ70MC62I8 |
nitryl chloride Chemical Properties,Uses,Production
Chemical Properties
Colorless gas; odor of chlorine; liquid and solutions have yellow tinge.
Physical properties
Colorless gas; chlorine-like odor; gas density 2.81 g/L at 100°C; liquefies to a pale-yellow liquid at -14.3°C; density of the liquid 1.33 g/mL; solidifies at -145°C; decomposes above 120°C; reacts with water.
Uses
Nitrating and chlorinating agent in organic syntheses.
Preparation
Nitryl chloride is prepared most conveniently by reacting chlorosulfonic acid with anhydrous nitric acid at 0°C:
An older preparation method involves passing dry chlorine gas slowly over dry silver nitrate heated to about 100°C. The gaseous reaction products are allowed to cool to low temperature. After several hours, nitryl chloride condenses to a pale yellowish-brown liquid. Chlorine is removed by purging with CO2.
2AgNO3 + 2Cl2 → 2NO2Cl + 2AgCl + O2.
Definition
ChEBI: Nitryl chloride is a nitro compound in which the nitrogen of the nitro group is bonded to a chlorine. It is used as a nitrating and chlorinating agent in organic synthesis. It has a role as an oxidising agent, an apoptosis inducer and a reagent. It is a nitro compound and a chlorine molecular entity.
Hazard
May explode on contact with organic materials. Corrosive to tissue.
Safety Profile
A poison by inhalation. Acorrosive irritant to skin, eyes, and mucous membranes. Apowerful oxidizer. The gas or liquid may attack organicmatter with explosive violence. Violent reaction withammonia or sulfur dioxide. Incompatible with tin(II)bromide
nitryl chloride Preparation Products And Raw materials
nitryl chloride Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8772 | 58 |
| Supplier | Advantage |
|---|---|
| Shaanxi Didu New Materials Co. Ltd | 58 |





