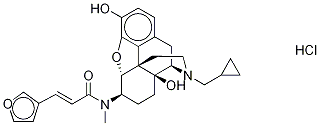
Nalfurafine
- CAS No.
- 152658-17-8
- Chemical Name:
- Nalfurafine
- Synonyms
- AC820;AC-820;AC 820;MT9938;MT 9938;MT-9938;Nalfurafina;TRK-820 HYDROCHLORIDE;Nafurarphine Hydrochloride;17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-epoxy-6beta-[N-methyl-trans-3-(3-furyl)acrylamido]morphinan hydrochloride
- CBNumber:
- CB72502851
- Molecular Formula:
- C28H33ClN2O5
- Molecular Weight:
- 513.02502
- MDL Number:
- MFCD09837714
- MOL File:
- 152658-17-8.mol
| Melting point | 207-217 °C |
|---|---|
| storage temp. | Store at -20°C |
| solubility | DMF: 16 mg/mL; DMSO: 33 mg/mL; Ethanol: 0.33 mg/mL; PBS (pH 7.2): 5 mg/mL |
| form | A solid |
| FDA UNII | 25CC4N0P8J |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H336-H302 | |||||||||
| Precautionary statements | P261-P271-P304+P340-P312-P403+P233-P405-P501-P264-P270-P301+P312-P330-P501 | |||||||||
| NFPA 704 |
|
Nalfurafine Chemical Properties,Uses,Production
Description
Pruritus (chronic itching) is a common symptom seen in 25-90% of
uremic patients, especially those with chronic renal failure requiring
hemodialysis.
Nalfurafine hydrochloride
is a new member of this class that exhibits an improved safety profile as
compared with its predecessors in preclinical studies. It is a potent
agonist for the κ-opioid receptor (Ki = 0.24 nM, EC50 = 0.008 nM, Imax =
91%), with substantially lower binding and agonism of the - or d-opioid
receptors (Ki = 2.24 and 484 nM, EC50 = 1.66 and 21.3 nM, Imax = 53 and
78%, respectively). In vivo, nalfurafine hydrochloride demonstrates
potent antipruritic activity against histamine-sensitive as well as histamine-resistant itch in mouse pruritogen-induced scratching models.
The most common adverse event associated with nalfurafine hydrochloride was insomnia or sleep disturbance, seen in 10% of the treated patients. Nalfurafine is structurally related to naltrexone (Revia ), an opioid receptor antagonist marketed for treating
alcohol dependence. Nalfurafine is synthesized in two steps starting from
naltrexone, via reductive amination with methylamine under catalytic
hydrogenation conditions, and subsequent acylation with 3(E)-(3-furyl)
acryloyl chloride.
Originator
Toray industries (Japan)
Uses
Nalfurafine hydrochloride was launched on March of 2009 in Japan as the first in class non-narcotic opioid drug for intractable itch caused by hemodialysis. It showed significant opioid κ-agonist activity and induced neither aversion nor preference in rats on the CPP (Conditioned Place Preference) test. A new therapeutic agent for the treatment of uremic pruritus in hemodialysis patients.
brand name
Remitch
Nalfurafine Preparation Products And Raw materials
Raw materials
Preparation Products
152658-17-8(Nalfurafine)Related Search:
1of4