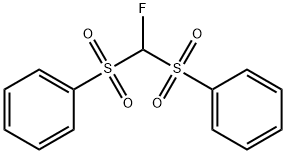
Fluorobis(phenylsulfonyl)methane
- CAS No.
- 910650-82-7
- Chemical Name:
- Fluorobis(phenylsulfonyl)methane
- Synonyms
- Bis(phenylsulfonyl)fluoromethane;Fluorobis(phenylsulfonyl)methane;(Fluoromethylenedisulfonyl)dibenzene;1,1'-[(fluoromethylene)bis(sulfonyl)]bis-benzene;1,1'-[(Fluoromethylene)bis(sulfonyl)]bis-benzene;Benzene, 1,1'-[(fluoromethylene)bis(sulfonyl)]bis-
- CBNumber:
- CB72537976
- Molecular Formula:
- C13H11FO4S2
- Molecular Weight:
- 314.35
- MDL Number:
- MFCD11036372
- MOL File:
- 910650-82-7.mol
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P |
| Safety Statements | 24/25 |
| HS Code | 29350090 |
Fluorobis(phenylsulfonyl)methane Chemical Properties,Uses,Production
Description
Fluorobis(phenylsulfonyl)methane (FBSM), can be deprotonated under much milder basic conditions than those required for the deprotonation of fluoromethyl phenyl sulfone, and thus has been used as an excellent nucleophilic fluoromethylation reagent in many catalytic asymmetric reactions with allyl esters, imines, and α,β-unsaturated compounds. Stereoselctive nucleophilic substitution reaction between chiral alcohols and FBSM under Mitsunobu conditions gives the fluoromethylated products with full inversion of the configuration. Nucleophilic substitution reaction of epoxides and aziridines with FBSM gives the precursors of β-fluoromethylated alcohols and amines in high yields. As a carbon acid, FBSM can also be used in cross dehydrogenative coupling reaction.
Uses
(Fluoromethylenedisulfonyl)dibenzene is a nucleophilic fluoromethylating agent.
Reactions
(1) Monofluoromethylation of allyl esters.
(2) Monofluoromethylation of Morita–Baylis–Hillman carbonates.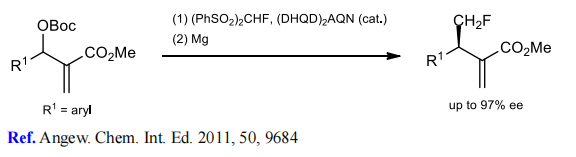
(3) Monofluoromethylation of imines.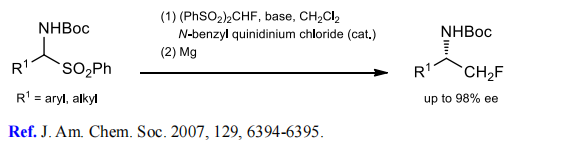
(4) Monofluoromethylation of α,β-unsaturated ketones and aldehydes.
(5) Monofluoromethylation of α,β-unsaturated ketones and aldehydes.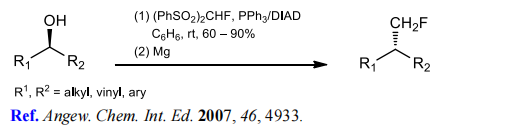
(6) Monofluoromethylation of epoxides.
(7) Monofluoromethylation of aldehydes.
(8) Monofluoromethylation of tertiary amines.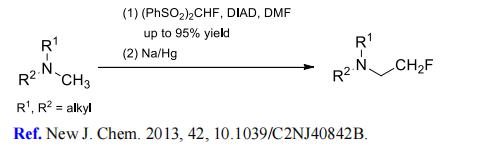
References
[1] HYOUNG WOOK MOON; Dae Y K; Min Je Cho. Enantioselective conjugate addition of fluorobis(phenylsulfonyl)methane to α,β-unsaturated ketones catalyzed by chiral bifunctional organocatalysts[J]. Tetrahedron Letters, 2009. DOI:10.1016/j.tetlet.2009.06.056.
[2] XIAOMING ZHAO. ChemInform Abstract: Highly Regioselective Pd-Catalyzed Allylic Alkylation of Fluorobis(phenylsulfonyl)methane[J]. ChemInform, 2011. DOI:10.1002/chin.201118076.
[3] G. K. SURYA PRAKASH. Efficient Nucleophilic Fluoromethylation and Subsequent Transformation of Alkyl and Benzyl Halides Using Fluorobis(phenylsulfonyl)methane[J]. Organic Letters, 2009. DOI:10.1021/ol8029627.
Fluorobis(phenylsulfonyl)methane Preparation Products And Raw materials
Raw materials
Preparation Products
Fluorobis(phenylsulfonyl)methane Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Allfluoro pharmaceutical co. ltd. | 021-26137118 +8615821363818 | sales@allfluoro.com | China | 5790 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10986 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Daicel Chiral Technologies (China)CO.,LTD | 021-50460086-9 15921403865 | han_yajun@dctc.daicel.com | China | 7189 | 65 |
| Shanghai T&W Pharmaceutical Co., Ltd. | +86 21 61551611 | China | 9891 | 58 | |
| Shanghai QianYan Bio-technology Co., Ltd | 02781293128 | orders@biochemsafebuy.com | China | 9923 | 55 |
| Greenherbs Science and Technology Development Co., Ltd. | 010-61136123 | pub02@greenherbs.com.cn | China | 4049 | 58 |
| JinJin Le Chemical Co., Ltd | 10106090 | jjlchem2@163.com | China | 9981 | 58 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 +86-13028896684; | sales@rrkchem.com | China | 57302 | 58 |
| Henan Alpha Chemical Co., Ltd. | 0371-0371-55055611 18137792234 | 3002694073@qq.com | China | 10527 | 58 |