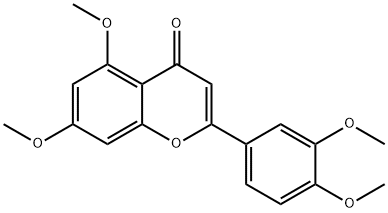
Luteolin
- CAS No.
- 491-70-3
- Chemical Name:
- Luteolin
- Synonyms
- 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4h-1-benzopyran-4-on;LUTEOLOL;luteoline;cyanidenon;digitoflavone;Rose extract P.E;uteoL;tgrtg;weldlake;Luteloin
- CBNumber:
- CB7282616
- Molecular Formula:
- C15H10O6
- Molecular Weight:
- 286.24
- MDL Number:
- MFCD00017309
- MOL File:
- 491-70-3.mol
- MSDS File:
- SDS
| Melting point | ~330 °C(lit.) |
|---|---|
| Boiling point | 348.61°C (rough estimate) |
| Density | 1.2981 (rough estimate) |
| refractive index | 1.4413 (estimate) |
| storage temp. | 2-8°C |
| solubility | Methanol (Slightly, Heated) |
| form | powder |
| pka | 6.50±0.40(Predicted) |
| color | yellow |
| Water Solubility | Soluble in aqueous alkaline solutions (1.4 mg/ml), ethanol (~5 mg/ml), dimethyl sulfoxide (7 mg/ml), 1eq. Sodium hydroxide (5 mM), dimethylformamide (~20 mg/ml), water (1 mg/ml) at 25°C and methanol. |
| Merck | 14,5614 |
| BRN | 292084 |
| InChIKey | IQPNAANSBPBGFQ-UHFFFAOYSA-N |
| LogP | 2.40 |
| CAS DataBase Reference | 491-70-3(CAS DataBase Reference) |
| EWG's Food Scores | 4 |
| FDA UNII | KUX1ZNC9J2 |
| NCI Drug Dictionary | luteolin |
| EPA Substance Registry System | Luteolin (491-70-3) |
| UNSPSC Code | 85151701 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-36/37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LK9275210 | |||||||||
| HS Code | 29329990 | |||||||||
| NFPA 704 |
|
Luteolin price More Price(65)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 440025 | Luteolin An antioxidant flavonoid and a free radical scavenger. | 491-70-3 | 5mg | $99.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 03880590 | Luteolin primary reference standard | 491-70-3 | 25mg | $486 | 2024-03-01 | Buy |
| TCI Chemical | T2682 | 3',4',5,7-Tetrahydroxyflavone >98.0%(HPLC) | 491-70-3 | 1g | $114 | 2024-03-01 | Buy |
| TCI Chemical | T2682 | 3',4',5,7-Tetrahydroxyflavone >98.0%(HPLC) | 491-70-3 | 5g | $446 | 2024-03-01 | Buy |
| Alfa Aesar | L14186 | 3',4',5,7-Tetrahydroxyflavone, 97% | 491-70-3 | 100mg | $80.9 | 2024-03-01 | Buy |
Luteolin Chemical Properties,Uses,Production
Natural Flavonoids
Luteolin is a very typical kind of natural flavonoid and belongs to weak acidic tetrahydroxy flavonoids. It is widely distributed in the plant kingdom and is mainly presented in honeysuckle, chrysanthemum, Nepeta, Herba Ajuga and some other drugs as well as many kinds of vegetables such as thyme, Brussels sprouts, cabbage, cauliflower, beets, broccoli and carrots. Moreover, it is also distributed in the form of glycosides in celery, green pepper, and basil leaves as well as the fruit shell of the legume plant Arachis hypogaea, Ajuga decumbus, Lonicera japonica Thunb, Gentianaceae plant Gentianopsis paludosa, and Valerianaceae plant Valeriana amurensis Smir. The pure product of Luteolin appears as yellow crystalline powder.

Figure 1 the pale yellow powder of luteolin and its sources of plant.
Solubility
Luteolin is a golden needles product containing a crystal water molecule that is precipitated from ethanol. It is soluble in alcohol and diethyl ether; slightly soluble in hot water, and insoluble in cold water. Its aqueous solution exhibits pleasing yellow and can be dissolved in 10% aqueous solution of sodium hydroxide and appears as dark yellow color. It is stable under normal conditions.
Extraction Method
According to the report of the literature, method for extracting the active ingredient luteolin from the peanut shell includes solvent method, ultrasonic method, microwave method and supercritical CO2 method, wherein the solvent extraction method is the most widely used with the major extraction solvents used being methanol, acetone and ethyl acetate.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Pharmacological activity
1. Luteolin has various kinds of pharmacological activities. Plants rich in luteolin content are often used as a traditional Chinese medicine for treatment of disease. With the deepening of the study on luteolin, researchers have found that it have anti-cancer activities including inhibiting the proliferation of tumor cell, inducing the apoptosis of tumor cells as well as sensitizing anti-cancer drugs. Moreover, it also has anti-inflammatory, anti-oxidant as well as protein effect on the nervous system.
2. Luteolin is a kind of PDE4 inhibitors, phosphodiesterase inhibitors (2) and interleukin-6 inhibitor (3). It can significantly reverse the induction effect of anesthesia of mice xylazine/ ketamine induction of anesthesia. 4 Preclinical studies have shown that the pharmacological effects of luteolin might include anti-oxidant, anti-inflammatory, antibacterial and anti-cancer. Preliminary studies have found that luteolin can inhibit the apoptosis of the angiogenesis induced cell, affect the tumor growth in animal models, reduce the tumor growth and also improve the cytotoxicity of certain anti-cancer drugs on the tumor cell, indicating that luteolin could be potential cancer chemo-preventive drugs and chemotherapy drugs.
3. The mechanism of biological activity of luteolin may be regulating the level of ROS, inhibition of topoisomerase I and topoisomerase II, reducing the transcription factor NF-κB and AP-1, and stabilizing p53 and inhibition of phosphatidylinositol 3-kinase, signal transduction and activator of transcription 3 (STAT3), insulin-like growth factor 1 receptor (IGF1R) and human epidermal growth factor receptor II activity.
Pharmacological effects
1, anti-tumor: the inhibitory effect of luteolin on tumor cell proliferation is mainly through inhibiting certain intracellular kinase activity and causing cell cycle arrest.
2. Antioxidant: the antioxidant effect of luteolin itself mainly exhibits as a reducing agent involved in the oxidation reaction as well as enhancing the activity of the biological antioxidant system.
3, Anti-inflammatory: the anti-inflammatory activity of luteolin is mainly manifested that it can reduce the activity of inflammatory cytokine transcription factor and production of the pro-inflammatory cytokines and inflammatory mediators.
4, the neuro-protective effect: luteolin has protective effect on the learning and memory ability of the nervous system.
5, anti-fibrosis: luteolin can reduce the extent of liver fibrosis, reduce the hydroxyproline (HYP) in the liver tissue, the content of malondialdehyde (MDA) and the mRNA expression of type I pro-collagen mRNA. In vitro, it can inhibit the proliferation of hepatic star like cells (HSC) and collagen synthesis. It can also alleviate the bleomycin-induced pulmonary fibrosis and histopathological changes, reduce the lung weight index, significantly suppress the increase of MDA, HYP and inhibit the expression of transforming growth factor-β1 (TGF-β1) mRNA in the lung tissue. In vitro, it can inhibit the proliferation of human embryo lung fibroblasts, induce the apoptosis.
6, anti-fertility and hormonal effects: luteolin has a significant dose-dependent anti-implantation activity. After oral administration, it can significantly increase the weight, diameter of uterine, the thickness of endometrium and the height of epithelial cells. Single administration has estrogenic effect while its combination with ethinyloestradiol exhibits anti-estrogenic effect.
7, other functions: luteolin can inhibit various kinds of bacteria and viruses, such as Staphylococcus aureus, Escherichia coli, herpes simplex virus, polio virus, Coxsackie B3 virus. It can inhibit the activity of integrase of AIDS virus HIV-1 and therefore has potential anti-HIV effect. Luteolin is able to bind to the s2 protein of the severe acute respiratory syndrome (SARS) corona virus, thus inhibiting viral for entry into host cells. Luteolin also has inhibitory effect on the Leishmania donovani. Through inhibiting the action of the topoisomerase I and topoisomerase II of Leishmania donovani and inhibit their growth. Additionally, luteolin also has immunomodulatory effects and so on.
Pharmacokinetics
The pharmacokinetic experiment of rat has shown that after the oral administration of luteolin by rats, the in vivo plasma concentration is significantly higher than that after acid hydrolysis which demonstrating it is mostly presented in the glucuronic acid-bound form. The biliary study have found that the level of luteolin in the biliary sample is undetectable using HPLC method at each time after the administration if without the hydrolysis treatment, demonstrating that luteolin is mainly presented in the bound form in the bile.
Indications
This product has antitussive, expectorant effect. The antitussive effect is through suppressing the cough center; the expectorant effect of this product is related to its ability of promoting the secretion of the respiratory tract gland and dissolving the acidic mucopolysaccharides in the sputum. It also has anti-inflammatory, anti-allergic and immune enhancement effect. It also has inhibitory effect on Staphylococcus aureus, Streptococcus pneumoniae and Pseudomonas aeruginosa. It can also be used for the treatment of chronic bronchitis and other respiratory diseases sputum.
Side effects
Some patients may get dry mouth, upset stomach, dizziness, nausea and other side effects. After prolonged administration, the symptom may gradually disappear.
Description
Luteolin is a flavone derived from Honeysuckle(Lonicera japonica Thunb). Luteolin is widely distributed in nature. It can be isolated from a variety of natural herbs, vegetables, and fruits. At present, luteolin is found mainly in honeysuckle, chrysanthemum, Schizonepeta, Ajuga, artichokes, Scutellaria, and Callicarpa nudiflora natural herbs. Dietary sources include celery, broccoli, green pepper, parsley, thyme, dandelion, perilla, chamomile tea, carrots, olive oil, peppermint, rosemary, navel oranges, and oregano. It can also be found in the seeds of the palm Aiphanes aculeata.
Chemical Properties
Yellow Needles
Physical properties
Appearance: yellow needle crystal. Solubility: slightly soluble in water and soluble in alkaline solution (monohydrate). Density, 1.654 g/cm3. Melting point, 330 °C. Boiling point, 616.1 °C (760 mmHg). Flash point, 239.5 °C. Vapor pressure, 9.03E-16 mmHg (25 °C). Acidity, weak acid.
History
At present, luteolin does not have the application of the proprietary medicine, but as
one of the main active ingredients of medicinal plants, there is a long history of
application. In northern and southern dynasties, honeysuckle with sweet taste, nontoxic, can treat swelling, lose weight, and prolong life under long-term use. In Tang dynasty, honeysuckle was used for treatment of abdominal distension, hot toxic blood dysentery, and water dysentery. It was showed that the clinical application of honeysuckle had made significant progress.
During the Song and Yuan dynasties, honeysuckle was widely used for the treatment of diseases such as sore and ulcer. To the Ming dynasty, there were many treatises about honeysuckle. For example, it is said in Compendium of Materia Medica: honeysuckle cure all rheumatism QI and all sorts of swollen poison, ulcer,scab, and heat dissipation detoxify. The prescription has also expanded its scope of application. Up to the Qing dynasty, the application of honeysuckle can not only inherit the theory of the predecessors but also put forward some original ideas and innovation in some respects.
In recent years, through the in-depth study of pharmacological effects, it is found that luteolin has significant effects on antitumor, cardioprotection, neuroprotection,respiratory system, immune regulation, anti-inflammatory, spasmolysis, expectorant, anti-allergic, enzyme activities, antioxidant, diuretic, and other aspects.
Uses
Luteolin has been used:
- to induce and elucidate the apoptotic pathway in renal cell carcinoma 786-O cells
- as an additive in M9 minimal medium to induce nodF gene expression
- as a reference standard to qualitatively and quantitatively analyse luteolin using reverse phase-high performance liquid chromatography with diode array detector (RP-HPLC-DAD)
- as a reaction supplement for β-galactosidase assay
- to elucidate the anti-inflammatory efficacy of luteolin in pseudorabies virus infected RAW264.7 cell line by measuring the anti-inflammatory mediators production and also cell viability and cytotoxicity assay
Definition
ChEBI: Luteolin is a tetrahydroxyflavone in which the four hydroxy groups are located at positions 3', 4', 5 and 7. It is thought to play an important role in the human body as an antioxidant, a free radical scavenger, an anti-inflammatory agent and an immune system modulator as well as being active against several cancers. It has a role as an EC 2.3.1.85 (fatty acid synthase) inhibitor, an antineoplastic agent, a vascular endothelial growth factor receptor antagonist, a plant metabolite, a nephroprotective agent, an angiogenesis inhibitor, a c-Jun N-terminal kinase inhibitor, an anti-inflammatory agent, an apoptosis inducer, a radical scavenger and an immunomodulator. It is a 3'-hydroxyflavonoid and a tetrahydroxyflavone. It is a conjugate acid of a luteolin-7-olate.
Indications
Luteolin compound prescription is mainly used for relieving cough, eliminating phlegm, diminishing inflammation, treating cardiovascular diseases, and treating amyotrophic lateral sclerosis, severe acute respiratory syndrome (SARS), hepatitis, etc.
General Description
Luteolin is a naturally occurring flavone, readily present in vegetables. It may possess many biological properties like anti-tumor activity against condition of skin papilloma. Luteolin is one of the most potent flavanoid inhibitors of soybean and reticulocyte 15-lipoxygenases, with an IC50 of 0.6 μM. Luteolin has also been found to inhibit the release of TNFα from neutrophils, and to inhibit matrix metalloproteinases.
Biological Activity
Anti-inflammatory, antioxidant and free radical scavenger. Inhibits LPS-induced TNF- α , IL-6 and inducible nitric oxide production and blocks NF- κ B and AP-1 activation. Antiproliferative; inhibits proliferation of Lewis lung carcinoma cells in vivo .
Biochem/physiol Actions
Hydroxylated flavone derivative, a strong antioxidant and radical scavenger. Suggested to play a role in prevention of cancer, possibly via the inhibition of fatty acid synthase activity.
Pharmacology
Luteolin can selectively inhibit the fatty acid synthase activity in prostate cancer and breast cancer cells, which is related to the inhibitory effect of luteolin on tumor cell growth and apoptosis. Luteolin can significantly reduce the incidence of colon cancer and the size of tumor caused by dimethylhydrazine, which may be related to the
regulation of lipid peroxidation, antioxidation, and antiproliferative effect.
The anti-inflammatory activity of luteolin is related to the inhibition of nitric oxide (NO) and other inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) generation and inhibition of protein tyrosine phosphorylation and nuclear transcription factor KB (NF-KB)-mediated gene expression.
Luteolin can enhance the transfer of synapses in the hippocampus dentate gyrus, causing long-term potentiation. Moreover, in chronic hypoperfusion injury caused by vascular occlusion, luteolin can still protect synapses, causing long-term potentiation, and reduce the escape latency in the Morris water maze test in rats.
Luteolin can reduce the degree of hepatic fibrosis, hydroxyproline level in liver tissues (HYP), malondialdehyde (MDA) content, and mRNA expression of procollagen type I and inhibit hepatic stellate cell (HSC) proliferation and collagen synthesis in vitro. Luteolin can also improve the histological changes of pulmonary fibrosis induced by bleomycin, reduce the lung weight index, significantly reduce the increase in MDA and HYP, and inhibit the level of mRNA of transforming
growth factor beta 1 (TGF-β1) in lung tissue. Luteolin in vitro can inhibit the proliferation of human embryonic lung fibroblast cells and promote apoptosis .
Anticancer Research
It is a flavone with yellow crystalline appearance. Dietary sources of luteolin includeoregano, celery, orange, broccoli, rosemary, green pepper, peppermint, parsley,olive oil, thyme, carrot, dandelion, chamomile tea, and perilla. It is found to obstructepithelial-mesenchymal transition (Singh et al. 2016b). It is inhibiting the cancercell proliferation, angiogenesis, and metastasis. In addition, it suppresses thepathways like PI3K/AKT, NF-κB, and X-linked inhibitor of apoptosis protein(XIAP) which enhances the cell growth and function. It also induces apoptosis andtumor suppressor p53. Hence, luteolin can be used as a potential antineoplasticagent in different cancers (Lin et al. 2008).
Clinical Use
The natural extract containing luteolin has been used in clinical treatment of many diseases. Lamiophlomis rotata Kudo capsule is made from traditional Chinese medicine Lamiophlomis rotata Kudo, which consists of the medicinal components such as flavonoids, saponins, sterols, amino acids, and many trace elements. Among these components, luteolin content is not less than 0.80 mg/g. This capsule is mainly used for a variety of surgical incision pain, postoperative bleeding, fracture, sprain of muscles, rheumatic pain, dysmenorrhea, uterine bleeding, gingival swelling, and bleeding.
storage
Store at +4°C
Luteolin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei baicao biology science and technology co., ltd | +86-19131911055 +8617824879454 | zhang@hbbocao.com | China | 1029 | 58 |
| PNP Biotech Co. Ltd | +8618516098983 | sales@pnpbiotech.com | China | 1001 | 58 |
| Rixing Chemical CO.,LTD. | +86-852-57055271 +8613237129059 | sales@rixingbiz.com | China | 232 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81139210 +86-18192627656 | 1059@dideu.com | China | 3003 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5870 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | mina@chuanghaibio.com | China | 18137 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3007 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | sales@zhswyy.com | China | 202 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20238 | 58 |
Related articles
- Luteolin: Overview and its Anticancer Perspectives
- Luteolin, a natural compound with antioxidant and anticancer properties, shows promise in inhibiting cancer progression in bre....
- Apr 30,2024
- Luteolin: activities and pharmacokinetics
- Luteolin is a bioactive flavonoid with anti-inflammatory, anti-cancer properties and pharmacokinetics enhanced by nanotechnolo....
- Dec 18,2023
- The effect of dietary Luteolin on glycolipid metabolism disorder
- Luteolin, as a natural compound with a wide range of biological activities, are an ideal dietary supplement for maintaining me....
- Nov 23,2023
View Lastest Price from Luteolin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-16 | Luteolin
491-70-3
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-04-16 | Luteolin
491-70-3
|
US $0.00-0.00 / kg | 1kg | 98 | 1000 | Wuhan Circle Star Chem-medical Technology Co.,Ltd | |
 |
2025-04-16 | Luteolin
491-70-3
|
US $0.00 / kg | 25kg | 99% | 1000kg | ZhenYiBio Technology Inc |
491-70-3(Luteolin)Related Search:
1of4