INSULIN
- CAS No.
- 11061-68-0
- Chemical Name:
- INSULIN
- Synonyms
- INSULIN, RECOMBINANT HUMAN;INSULIN HUMAN;rh-Insulin;Insulin (human) CRS;D03230;Umulin;novolin;Humulin;INSULIN;humuline
- CBNumber:
- CB7336087
- Molecular Formula:
- C257H383N65O77S6
- Molecular Weight:
- 0
- MDL Number:
- MFCD00131380
- MOL File:
- Mol file
| Melting point | >200°C (dec.) |
|---|---|
| storage temp. | -20°C |
| solubility | acidified water, pH 2.0: 2 mg/mL |
| form | solution |
| color | White |
| Water Solubility | Soluble up to 10mg/ml in pH <3 or in the presence of surfactants. Adjusting pH with a volatile acid (such as formic acid) prior to dry down will allow the product to be re-dissolved in water. |
| FDA UNII | 1Y17CTI5SR |
| ATC code | A10AB01,A10AC01,A10AD01,A10AE01,A10AF01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Safety Statements | 22-24/25 |
| WGK Germany | 3 |
| RTECS | NM8900250 |
| F | 3-10 |
| HS Code | 2937120000 |
| Toxicity | LD50 intraperitoneal in mouse: 3937units/kg |
INSULIN price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | I1507 | Insulin human ≥95% (HPLC), semisynthetic, powder | 11061-68-0 | 0.1mg | $780 | 2024-03-01 | Buy |
| Sigma-Aldrich | I1507 | Insulin human ≥95% (HPLC), semisynthetic, powder | 11061-68-0 | 0.5mg | $1112 | 2024-03-01 | Buy |
| Sigma-Aldrich | 407709 | Insulin, Zinc, Human, Recombinant, | 11061-68-0 | 50mg | $242 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1342106 | Insulin human United States Pharmacopeia (USP) Reference Standard | 11061-68-0 | 100mg | $510 | 2024-03-01 | Buy |
| Sigma-Aldrich | 11376497001 | Insulin, human recombinant (yeast) | 11061-68-0 | 100mg | $226 | 2024-03-01 | Buy |
INSULIN Chemical Properties,Uses,Production
Gene, mRNA, and precursor
The human preproINS gene, INS, is located at 11p15.5
and consists of three exons. In mice, it is located on chromosome 7. There are many regulatory elements, such as
A-box, GG box, and the cAMP response element, in the
promoter region. The coding region of human and tilapia
preproINS mRNAs comprises 330 and 339 bases, respectively. In humans, INS forms a gene cluster with IGF2 and H19, which is a gene for a long noncoding
RNA.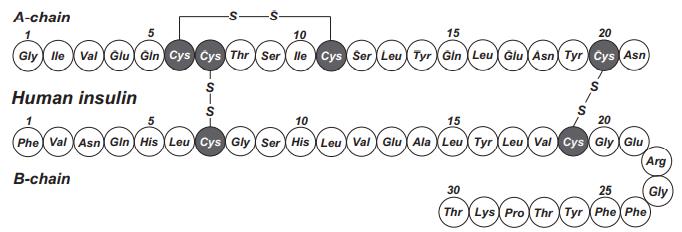
Regulation of synthesis
INS gene transcription is stimulated by glucose in mammals, and glucose treatment results in a 2- to 5-fold elevation within 60–90min. Long-term exposure to physiological concentrations of estradiol-17β increases the β-cell content, INS gene expression, and INS release in mice. Fasting reduces the expression levels of two INS genes in the trout.
Receptors
INS receptors (INSRs) are heterotetrameric glycoproteins containing many phosphorylated or glycosylated sites. INS binds to the two extracellular α-subunits linked by a disulfide bond. The two β-subunits are connected to the α-subunits by a disulfide bond and contain a tyrosine kinase domain in their intracellular region. Human INSR is encoded by a single gene, INSR, location 19p13.3-p13.2, comprising 22 exons.INSRencodes 1382 aa residues of the ISNR precursor, which contains a signal peptide and α- and β-subunits. Human INSR generates two alternative splicing variants (INSR-A and -B) that differ at the carboxyl terminus of their α-subunit. INSR-A lacks the terminus encoded by exon 11. INSR-B has not been detected in the chicken or Xenopus. There are three types of hybrid receptors among these INSRs and the IGF-I receptor (IGF-IR), and their expression ratio influences the INS action at the target organs. Dysregulation of the INSR-A/INSR-B ratio is associated with INS resistance, aging, and increased proliferative activity of normal and neoplastic tissues. The dissociation constants (Kd) of human INS to human INSR and IGF-1R are 0.23 and 16nM, respectively. IGF-I binds to hybrid receptors with at least a 50-fold higher affinity than INS, irrespective of the splicing variant. Guinea pig INSR shows an exceptionally higher affinity (Kd= 0.083nM). Several fish species have multiple INSR genes that show different expression patterns among tissues. Human INS binds to fish INSR at a Kd of 0.67 and 0.15nM in the trout and carp, respectively.
Agonists and Antagonists
IGFs bind to INSR, but INS binds to IGF-IR with low affinity. Several synthetic peptides (S519 and RB539) show agonistic effects with a high affinity to INSR. Fish INSs bind mammalian INSR with high affinity and vice versa. RB537, S661, and S961 are INSR antagonists with high affinity. S961 shows an agonistic effect only on 3H-thymidine incorporation
Biological functions
INS is a key regulator of glucose homeostasis in mammals because the peptide is the only hormone that lowers
blood glucose levels. INSR-A mainly enhances the
effects of IGF-II during embryogenesis and fetal development, whereas INSR-B is predominantly expressed in
adults and enhances the metabolic effects of INS. ProINS binds to INSR-A, which is present in the nervous
system, to elicit antiapoptotic and neuroprotective effects
in the developing and postnatal nervous system. In the
pancreas, the exocrine and endocrine components stimulate each other via intrapancreatic axes of communication. The insular-acinar axis is involved in the
regulation of pancreatic digestive enzyme production
in acinar cells located in the blood downstream of β-cells,
and the acini-insular axis is involved in the regulation of
INS release by pancreatic enzymes.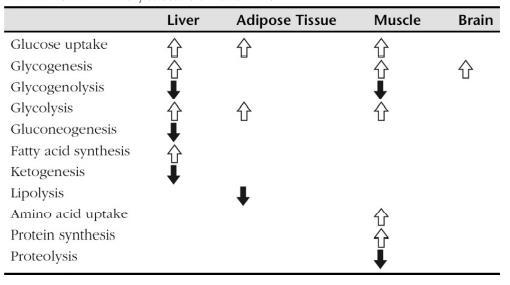
Clinical implications
Diabetes is classified as type 1 or type 2. Type 1 diabetes (INS-dependent diabetes mellitus) results from the autoimmune destruction of β-cells. Type 2 diabetes (noninsulin-dependent diabetes mellitus) is a metabolic disorder characterized by hyperglycemia due to an absolute or relative lack of INS or cellular resistance to INS. In mammals and fish, the administration of alloxan or streptozotocin selectively destroys β-cells, producing a type 2 diabetes model.
Description
Insulin (INS) is the only hormone lowering blood glucose levels in vertebrates. However, the importance of insulin in the carbohydrate metabolism may differ between mammals and other vertebrates. Frederick Banting and Charles Best discovered a substance that lowered blood sugar levels in the dog pancreas in 1921, and this was immediately applied to diabetes care. The primary structure of INS has been reported in many vertebrates, including agnathans, fish, and tetrapods.
Chemical Properties
White or almost white powder.
Uses
Antidiabetic.
Uses
Insulin shows a broad range of activities on a variety of somatic cells. Recombinant human insulin can be used to stimulate growth and proliferation of cultured cells and to investigate insulin activity on sensitive cells used in research studies. It is also a component of serum-free media formulations for most primary cells and cell lines.
brand name
Humulin (Lilly); Novolin (Novo Nordisk); Velosulin (Novo Nordisk).
General Description
The INS gene encodes for preproinsulin, which is enzymatically converted into insulin. Insulin is produced in the insulin-producing pancreatic β cells. Preproinsulin is converted to proinsulin in ER and proinsulin is then proteolytically processed to form insulin in newly-forming insulin secretory granules. Insulin production is tightly regulated by specific DNA elements present within ~400 bp in the proximal region of the INS promoter.
Biochem/physiol Actions
Insulin is responsible for two types of actions- excitatory and inhibitory. In its excitatory role, it increases the uptake of glucose and lipid synthesis, and in its inhibitory role it inhibits glycogenolysis, gluconeogenesis, lipolysis, proteolysis and ketogenesis. Aberrant insulin secretion leads to various disorders such as diabetes, hyperglycemia or hypoglycaemia. Type I diabetes is a result of autoimmune destruction of β cells of pancreas, which leads to depletion of insulin. Mutant INS-gene Induced Diabetes of Youth (MIDY) syndrome is an autosomal dominant disorder caused by missense mutations, which lead to aberrant proinsulin folding. Impaired glucose tolerance (IGT) or non-insulin-dependent diabetes mellitus (NIDDM) is caused by resistance to insulin-stimulated glucose uptake.
Clinical Use
In mammals and chickens, INS levels are routinely measured using commercial kits, and several studies have established specific immunoassays for fish. Many INS analogs, which show different durations of action, have been developed to treat diabetes. Alternative INS administration methods other than injection are via the lungs, nose, skin, and mouth. These have been developed using the insulin encapsulation technique with micro- or nanodelivery systems. Many medical drugs that stimulate INS release, both directly and indirectly, are commercially available. Sulfonylurea and glinides stimulate β-cells directly. The incretin GLP-1 analog can lower glucose concentrations by augmenting insulin secretion and suppressing glucagon release. In addition the dipeptidyl peptidase-4 (DPP-4) inhibitor inhibits incretin degradation and indirectly enhances the stimulation of incretin to β-cells. Thiazolidinedione improves the action of INS in the muscle and adipose tissue. Cone snail (Conus geographus) G1 is a naturally occurring B-chainminimized mimetic of INS, which strongly binds to human and fish INSR and activates receptor signaling. This peptide induces extremely rapid hypoglycemic shock.
in vitro
methotrexate (mtx) was found to be linked to insulin covalently. as effectively as insulin, insulin-mtx complex competed with 125i-insulin for insulin receptor. it was found that ic50 and ki for insulin-mtx were 93.82 nm and 91.88 nm, respectively, whereas those for insulin were 5.01 nm and 4.85 nm, respectively [1].
in vivo
previous study showed that insulin-stimulated glucose uptake in extensor muscles from sjl mice was reduced, but the basal uptake rates were not different. in another mouse study, knockdown of tc10α but not tc10β in 3t3-l1 adipocytes resulted in a inhibition of both insulin-stimulated glucose uptake and glut4 translocation [2].
IC 50
5.01 nmol/l for insulin receptor
storage
Store at -20°C
Structure and conformation
Human preproINS consists of 110 aa residues: 24 in the
signal peptide, 30 in the B-chain, 31 in the C-peptide, and
21 in the A-chain. The signal peptide and
C-peptide are excised from the preproINS to produce
the mature peptide, consisting of heterodimeric A- and
B-chains. Six cysteine residues are conserved throughout evolution from agnathans to mammals. Furthermore, the N-terminal seven aa residues of the A-chain
and the receptor binding region of the B-chain (Gly-Phe-Phe-Tyr) are highly conserved. Mr 5808, pI 5.3. INS is soluble under acidic and alkaline
conditions and almost insoluble at neutral pH. INS can be
stored for up to 6 months at 4°C in 1M acetic acid. Long-term
storage at an alkaline pH increases the rate of deamidation
and aggregation. One IU is equivalent to 0.0347mg
human INS.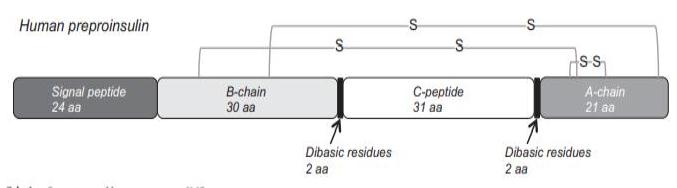
References
[1] ou x,kuang a,liang z,peng x,zhong y. the binding characteristics of insulin-mtx to insulin receptor. hua xi yi ke da xue xue bao.2001 dec;32(4):538-40.
[2] leney se,tavaré jm. the molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. j endocrinol.2009 oct;203(1):1-18.
INSULIN Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Nanjing Duly Biotech Co.,Ltd | +undefined18013301590 | melody@njduly.com | China | 2450 | 58 |
| Shanghai Getian Industrial Co., LTD | +86-15373193816 +86-15373193816 | mike@ge-tian.com | China | 269 | 58 |
| Shanghai Likang New Materials Co., Limited | +86-16631818819 +86-17736933208 | 3684455296@qq.com | China | 9300 | 58 |
| Binzhou Lista Trading Limited | sallychris2021@gmail.com | China | 74 | 58 | |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29883 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
Related articles
- The Synthesis and Sources of Insulin
- Over the years, the methods of insulin synthesis have evolved significantly, moving from extraction from animal sources to mor....
- Jul 25,2024
View Lastest Price from INSULIN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Insulin
11061-68-0
|
US $50.00-30.00 / box | 1box | 99.99% | 1000 | hebei hongtan Biotechnology Co., Ltd | |
 |
2024-11-19 | Insulin (human)
11061-68-0
|
US $30.00-62.00 / mg | 99.99% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-08-12 | Insuline
11061-68-0
|
US $150.00-135.00 / box | 1box | 99.99% | 200boxes | Binzhou Lista Trading Limited |
-

- Insulin
11061-68-0
- US $50.00-30.00 / box
- 99.99%
- hebei hongtan Biotechnology Co., Ltd
-

- Insulin (human)
11061-68-0
- US $30.00-62.00 / mg
- 99.99%
- TargetMol Chemicals Inc.
-

- Insuline
11061-68-0
- US $150.00-135.00 / box
- 99.99%
- Binzhou Lista Trading Limited




