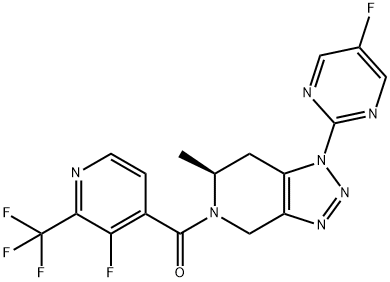
JNJ-55308942 (JNJ55308942)
- CAS No.
- 2166558-11-6
- Chemical Name:
- JNJ-55308942 (JNJ55308942)
- Synonyms
- JNJ-55308942 (JNJ55308942)
- CBNumber:
- CB74844940
- Molecular Formula:
- C17H12F5N7O
- Molecular Weight:
- 425.32
- MDL Number:
- MFCD34184759
- MOL File:
- 2166558-11-6.mol
JNJ-55308942 (JNJ55308942) Chemical Properties,Uses,Production
Biological Activity
JNJ-55308942 is a high-affinity, selective, brain-penetrant P2X7 functional antagonist (hP2X7: IC50=10 nM, Ki=7.1 nM; rP2X7: IC50=15 nM, Ki=2.9 nM). JNJ-55308942 is orally bioavailable, binds to brain P2X7 and blocks IL-1β release from adult rodent brain[1][2]. JNJ-55308942 shows pKis of 8.1and 8.5 for recombinant human and rat P2X7 channels, respectively. In human blood and in mouse blood and microglia, JNJ-55308942 attenuates IL-1β release in a potent and concentration-dependent manner[2]. JNJ-55308942 (30 mg/kg; p.o.) attenuates LPS-induced microglial activation in mice[2].In a model of Bacillus Calmette-Guerin (BCG)-induced depression, JNJ-55308942 dosed orally (30 mg/kg), reversed the BCG-induced deficits of sucrose preference and social interaction. After oral dosing, the compound exhibited both dose and concentration-dependent occupancy of rat brain P2X7 with an ED50 of 0.07 mg/kg. The P2X7 antagonist (3 mg/kg, oral) blocked Bz-ATP-induced brain IL-1β release in conscious rats, demonstrating functional effects of target engagement in the brain[2]. JNJ-55308942 (5 mg/kg; p.o.) shows the F, Vss, CL, Cmax and AUC24h values are 81%, 1.7 L/kg, 3.7 mL min/kg, 1747 ng/mL, and 17549 (ng/mL) h, respectively[1].
References
[1]. Bhattacharya A, et al. Neuropsychopharmacology of JNJ-55308942: evaluation of a clinical candidate targeting P2X7 ion channels in animal models of neuroinflammation and anhedonia. Neuropsychopharmacology. 2018;43(13):2586-2596. [2]. Chrovian CC, et al. A Dipolar Cycloaddition Reaction To Access 6-Methyl-4,5,6,7-tetrahydro-1H-[1,2,3]triazolo[4,5-c]pyridines Enables the Discovery Synthesis and Preclinical Profiling of a P2X7 Antagonist Clinical Candidate. J Med Chem. 2018;61(1):207-223.
JNJ-55308942 (JNJ55308942) Preparation Products And Raw materials
Raw materials
Preparation Products
JNJ-55308942 (JNJ55308942) Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Wuhan kemike Biomedical Technology Co., Ltd | 027-87747081 18186681184 | 1344475572@qq.com | China | 7243 | 58 |
| Shanghai Dexuan Pharmaceutical Technology Co., Ltd | 1701086315 | dexuanpharma@126.com | China | 9478 | 58 |
| Hubei Kele Fine Chemical Co., Ltd | 027-59101668 19945030958 | 2881924765@qq.com | China | 7969 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 23980 | 58 |
| Shanghai?Medlife?Pharm-Tech?Co.,?Ltd | 021-59167510 18117107507 | vip@med-life.cn | China | 5012 | 58 |
| RD International Technology Co., Limited | 18024082417 | market@ubiochem.com | China | 9268 | 58 |
| Nanjing Shizhou Biology Technology Co.,Ltd | 025-85560043 15850508050 | cindy.huang@synzest.com | China | 12007 | 58 |