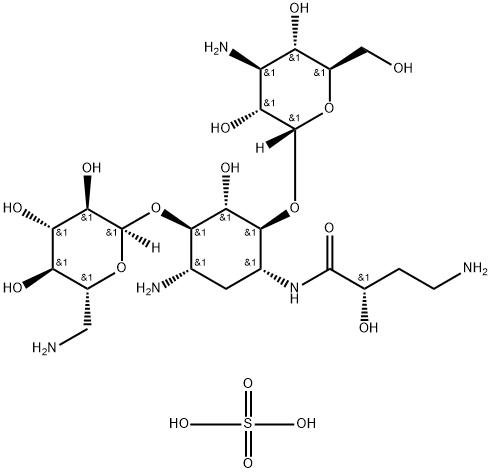
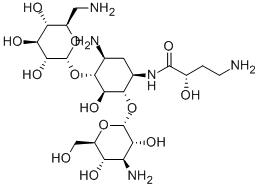
Amikacin Disulfate
- CAS No.
- 39831-55-5
- Chemical Name:
- Amikacin Disulfate
- Synonyms
- AMIKACIN SULFATE;AMIKACIN SULPHATE;Sterile;AMIKACIN DISULFATE SALT;AMika;Grasil;Cashimy;Biodacyn;Biodacyna;Selemycin
- CBNumber:
- CB7688755
- Molecular Formula:
- C22H45N5O17S
- Molecular Weight:
- 683.68
- MDL Number:
- MFCD00167475
- MOL File:
- 39831-55-5.mol
| Melting point | 220-230 C |
|---|---|
| alpha | D22 +74.75° (water) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | powder |
| color | white to off-white |
| PH | pH(10g/L, 25℃) : 2.0~4.0 |
| Water Solubility | Soluble in water at 50mg/ml with warming |
| Merck | 13,404 |
| BRN | 6172633 |
| Stability | Hygroscopic |
| CAS DataBase Reference | 39831-55-5(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | sterile |
| FDA UNII | N6M33094FD |
| Proposition 65 List | Amikacin Sulfate |
| EPA Substance Registry System | Amikacin sulfate (39831-55-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H303-H317-H318-H372 |
| Precautionary statements | P264b-P270-P272-P280-P302+P352-P305+P351+P338-P310-P363-P403-P501c |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 2 |
| RTECS | WK1961200 |
| F | 3-10 |
| HS Code | 29419000 |
| Toxicity | LD50 oral in rabbit: > 3gm/kg |
Amikacin Disulfate price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A1774 | Amikacin disulfate salt potency: 674-786μg per mg (as amikacin base) | 39831-55-5 | 1g | $183 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1019494 | Amikacin sulfate United States Pharmacopeia (USP) Reference Standard | 39831-55-5 | 100mg | $436 | 2024-03-01 | Buy |
| Alfa Aesar | J63862 | Amikacin disulfate | 39831-55-5 | 25g | $162 | 2024-03-01 | Buy |
| Alfa Aesar | J63862 | Amikacin disulfate | 39831-55-5 | 100g | $452 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0001117 | Amikacin for system suitability European Pharmacopoeia (EP) Reference Standard | 39831-55-5 | y0001117 | $229 | 2024-03-01 | Buy |
Amikacin Disulfate Chemical Properties,Uses,Production
Chemical Properties
White Solid
Originator
Amikin,Bristol,US,1976
Uses
Amikacin disulfate is used as an aminoglycoside antibiotic derived from Kanamycin A, used for treating infections with multidrug resistant Gram negative bacteria such as Pseudomonas aeruginosa, Acinetobacter, and Enterobacter. It is commonly used in the treatment of drug-resistant mycobacteria. It is used to study organism-directed delivery of antibiotics as well as drug resistance.
Uses
Analgesic
Uses
Semisynthetic aminoglycoside antibiotic derived from Kanamycin A. Antibacterial.
Definition
ChEBI: An aminoglycoside sulfate salt obtained by combining amikacin with two molar equivalents of sulfuric acid.
Manufacturing Process
Preparation of L-(-)-γ-benzyloxycarbonylamino-α-hydroxybutyric acid: L-(-)-γ-
amino-α-hydroxybutyric acid (7.4 g, 0.062 mol) was added to a solution of
5.2 grams (0.13 mol) of sodium hydroxide in 50 ml of water. To the stirred
solution was added dropwise at 0-5°C over a period of 0.5 hour, 11.7 grams
(0.068 mol) of carbobenzoxy chloride and the mixture was stirred for another
hour at the same temperature. The reaction mixture was washed with 50 ml
of ether, adjusted to pH 2 with dilute hydrochloric acid and extracted with four
80 ml portions of ether. The ethereal extracts were combined, washed with a
small amount of saturated sodium chloride solution, dried with anhydrous
sodium sulfate and filtered. The filtrate was evaporated in vacuum and the
resulting residue was crystallized from benzene to give 11.6 grams (74%) of
colorless plates; MP 78.5°C to 79.5°C.
Preparation of N-Hydroxysuccinimide Ester of L-(-)-γ-Benzyloxycarbonylamino-
α-hydroxybutyric acid: A solution of 10.6 grams (0.042 mol) of L-(-)-γ-
benzyloxycarbonylamino-α-hydroxybutyric acid and 4.8 grams (0.042 mol) of
N-hydroxysuccinimide in 200 ml of ethyl acetate was cooled to 0°C and then
8.6 grams (0.042 mol) of dicyclohexylcarbodiimide was added. The mixture
was kept overnight in a refrigerator. The dicyclohexylurea which separated
was filtered off and the filtrate was concentrated to about 50 ml under
reduced pressure to give colorless crystals of L-(-)-γ-benzyloxycarbonylamino-
α-hydroxybutyric acid which were collected by filtration; 6.4 grams, MP 121-
122.5°C. The filtrate was evaporated to dryness in vacuum and the crystalline
residue was washed with 20 ml of a benzene-n-hexane mixture to give an
additional amount of L-(-)-γ-benzyloxycarbonylamino-α-hydroxybutyric acid.
The total yield was 13.4 grams (92%).
Preparation of 1-[L-(-)-γ-Benzyloxycarbonylamino-α-Hydroxybutyryl]-6'-
Carbobenzoxykanamycin A: A solution of 1.6 grams (4.6 mmol) of L-(-)-γ-
benzyloxycarbonylamino-α-hydroxybutyric acid in 40 ml of ethylene glycol
dimethyl ether (DME) was added dropwise to a stirred solution of 2.6 grams
(4.2 mmol) of 6'-monobenzyloxycarbonylkanamycin A in 40 ml of 50%
aqueous ethylene glycol dimethyl ether and the mixture was stirred overnight.
The reaction mixture was evaporated under reduced pressure to give a brown
residue 1-[L-(-)-γ-benzyloxycarbonylarnino-α-hydroxybutyryl]-6'-
carbobenzoxykanamycin A which was used for the next reaction without
further purification.
Preparation of 1-[L-(-)-γ-Amino-α-Hydroxybutyryl] Kanamycin A: The crude
product 1-[L-(-)-γ-benzyloxycarbonylamino-α-hydroxybutyryl]-6'-
carbobenzoxykanamycin A was dissolved in 40 ml of 50% aqueous dioxane
and a small amount of insoluble material was removed by filtration. To the
filtrate was added 0.8 ml of glacial acetic acid and 1 gram of 10% palladiumon-
charcoal and the mixture was hydrogenated at room temperature for 24
hours in a Parr hydrogenation apparatus. The reaction mixture was filtered to
remove the palladium catalyst and the filtrate was evaporated to dryness in
vacuum.
The residue was dissolved in 30 ml of water and chromatographed on a column of CG-50 ion exchange resin (NH4
+ type, 50 cm x 1.8 cm). The
column was washed with 200 ml of water and then eluted with 800 ml of 0.1
N NH4OH, 500 ml of 0.2 N NH4OH and finally 500 ml of 0.5 N NH4OH. Ten
milliliter fractions were collected and fractions 146 to 154 contained 552 mg
(22%. based on carbobenzoxykanamycin A, 6'-
monobenzyloxycarbonylkanamycin A) of the product which was designated
BB-K8 lot 2. MP 187°C (dec). Relative potency against B. subtilis (agar plate)
= 560 mcg/mg (standard: kanamycin A free base).
A solution of 250 mg of BB-K8 lot 2 in 10 ml of water was subjected to
chromatography on a column of CG-50 (NH4
+ type, 30 cm x 0.9 cm). The
column was washed with 50 ml of water and then eluted with 0.2 N NH4OH.
Ten milliliter fractions were collected. Fractions 50 to 63 were combined and
evaporated to dryness under reduced pressure to give 98 mg of the pure
product base.
Preparation of the Monosulfate Salt of 1-[L-(-)-γ-Amino-α-Hydroxybutyryl]
Kanamycin A: One mol of 1-[L-(-)-γ-amino-α-hydroxybutyryl] kanamycin A is
dissolved in 1 to 3 liters of water. The solution is filtered to remove any
undissolved solids. To the chilled and stirred solution is added one mol of
sulfuric acid dissolved in 500 ml of water. The mixture is allowed to stir for 30
minutes, following which cold ethanol is added to the mixture till precipitation
occurs. The solids are collected by filtration and are determined to be the
desired monosulfate salt.
brand name
Amikin (Apothecon).
Therapeutic Function
Antibacterial
Safety Profile
Poison by intravenous route.Moderately toxic by intraperitoneal and subcutaneousroutes. An experimental teratogen. Other experimentalreproductive effects. When heated to decomposition itemits very toxic fumes of NOx and SOx.
Amikacin Disulfate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12837 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20285 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8615713292910 | Nancy@kangcang.com.cn | China | 341 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
View Lastest Price from Amikacin Disulfate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-13 | Amikacin Disulfate
39831-55-5
|
US $0.00 / KG/Tin | 6.5KG | 1:1.8 salt; 691-806 μg/mg; Sterile; USP | 10 TONS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-12-09 | AMIKACIN SULFATE
39831-55-5
|
US $0.00-0.00 / KG | 1KG | 99% | 1000 MT | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-11-19 | Amikacin disulfate
39831-55-5
|
US $42.00 / mg | 99.88% | 10g | TargetMol Chemicals Inc. |
-

- Amikacin Disulfate
39831-55-5
- US $0.00 / KG/Tin
- 1:1.8 salt; 691-806 μg/mg; Sterile; USP
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- AMIKACIN SULFATE
39831-55-5
- US $0.00-0.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Amikacin disulfate
39831-55-5
- US $42.00 / mg
- 99.88%
- TargetMol Chemicals Inc.