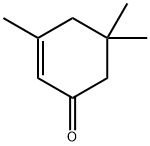
Isophorone
- CAS No.
- 78-59-1
- Chemical Name:
- Isophorone
- Synonyms
- Izoforon;3,5,5-TRIMETHYL-2-CYCLOHEXEN-1-ONE;Isophoron;3,5,5-trimethylcyclohex-2-en-1-one;IPHO;Isoforon;FEMA 3553;Isoforone;isooctopherone;3,5,5-TRIMETHYL-2-CYCLOHEXENONE
- CBNumber:
- CB7743348
- Molecular Formula:
- C9H14O
- Molecular Weight:
- 138.21
- MDL Number:
- MFCD00001584
- MOL File:
- 78-59-1.mol
- MSDS File:
- SDS
| Melting point | -8 °C (lit.) |
|---|---|
| Boiling point | 213-214 °C (lit.) |
| Density | 0.923 g/mL at 25 °C (lit.) |
| vapor density | 4.77 (vs air) |
| vapor pressure | 0.2 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 3553 | ISOPHORONE |
| Flash point | 184 °F |
| storage temp. | Store below +30°C. |
| solubility | 14.5g/l |
| form | Liquid |
| color | Clear colorless to yellow |
| Odor | Like camphor. |
| explosive limit | 0.8-3.8%(V) |
| Odor Type | woody |
| biological source | synthetic |
| Viscosity | 2.83mm2/s |
| Water Solubility | Soluble in water (12g/L). |
| Merck | 14,5196 |
| JECFA Number | 1112 |
| BRN | 1280721 |
| Henry's Law Constant | (x 10-6 atm?m3/mol): 5.8 (calculated, U.S. EPA, 1980a) |
| Exposure limits | TLV-TWA 25 mg/m3 (5 ppm); IDLH 800 ppm. |
| Stability | Stable. Substances to be avoided include strong bases, strong acids and strong oxidizing agents. |
| LogP | 1.67-1.7 at 20℃ |
| Substances Added to Food (formerly EAFUS) | ISOPHORONE |
| FDA 21 CFR | 175.105 |
| CAS DataBase Reference | 78-59-1(CAS DataBase Reference) |
| FDA UNII | 2BR99VR6WA |
| NIST Chemistry Reference | 2-Cyclohexen-1-one, 3,5,5-trimethyl-(78-59-1) |
| EPA Substance Registry System | Isophorone (78-59-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312-H319-H335-H351 | |||||||||
| Precautionary statements | P201-P280-P301+P312-P302+P352+P312-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 21/22-36/37-40 | |||||||||
| Safety Statements | 13-23-36/37/39-46 | |||||||||
| RIDADR | UN 3082 9 / PGIII | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 4 ppm (23 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GW7700000 | |||||||||
| Autoignition Temperature | 864 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2914 29 00 | |||||||||
| Hazardous Substances Data | 78-59-1(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in male, female rats and male mice (mg/kg): 2700 ±200, 2100 ±200, 2200 ±200 orally (PB90-180225) | |||||||||
| IDLA | 200 ppm | |||||||||
| NFPA 704 |
|
Isophorone price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | I18709 | Isophorone 97% | 78-59-1 | 5ml | $24.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | I18709 | Isophorone 97% | 78-59-1 | 1l | $63.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.04807 | Isophorone for synthesis | 78-59-1 | 100mL | $27.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.04807 | Isophorone for synthesis | 78-59-1 | 1L | $64.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.04807 | Isophorone for synthesis | 78-59-1 | 2.5L | $130 | 2024-03-01 | Buy |
Isophorone Chemical Properties,Uses,Production
Description
Isophorone (chemical formula: C9H14O) has its full name being 3, 5, 5-Trimethyl-2-cyclohexen-1-one. It is a kind of α, β-unsaturated cyclic ketone, and is a flavoring ingredient existing in cranberries and saffron. Isophorone can be used as a solvent in some printing inks, paints, lacquers, adhesives, copolymers, coatings, finishings and pesticide. It can also act as an intermediate in organic synthesis as well as the ingredient in wood preservatives and floor sealants. It is mainly manufactured through the self-condensation of acetone with KOH.
Chemical Properties
Isophorone, an alpha, beta-unsaturated ketone, is also an industrial solvent used in a variety of different applications. Most of the isophorone used in industry is used in vinyl coatings and inks.

Isophorone is an insoluble, colorless to white liquid and has an odor similar to peppermint. Its characteristic odor is detectable at concentrations as low as 1.00 mg/m3 (0.2 ppm) (Ruth, 1986). Although not highly volatile, with a vapor pressure of 0.438mm Hg at 25 °C.
References
https://en.wikipedia.org/wiki/Isophorone
https://pubchem.ncbi.nlm.nih.gov/compound/isophorone#section=Top
Chemical Properties
Isophorone is a colorless low volatility liquid that smells like peppermint. It is miscible with most organic solvents and can dissolve 1.2g in 100g of water. When exposed to light, it becomes a dimer, which is oxidized in air to generate 4,4,6-Trimethyl-1,2-cyclohexanedione.
Physical properties
Clear, colorless liquid with a sharp peppermint or camphor-like odor. Experimentally determined detection and recognition odor threshold concentrations were 1.1 mg/m3 (190 ppbv) and 3.0 μg/m3 (530 ppbv), respectively (Hellman and Small, 1974).
Occurrence
Reported found in Burley tobacco, cranberry, macadamia nuts, peas, roasted filbert, saffron, wine, osmanthus, grapefruit juice, papaya, kohlrabi, Parmesan cheese, roast beef, black tea, oats, Japanese plum, prunes, plumcot, starfruit, mango, rice, buckwheat, okra and sweet grass oil.
Uses
Isophorone is used as a solvent for vinylresins and cellulose esters, and in pesticides. solvent in some printing inks, paints, lacquers and adhesives. As a cyclic unsaturated ketone, isophorone can be aromatized to 3,5-dimethylphenol or 2,3,5-trimethylphenol. Hydrogenation leads, depending on conditions, to 3,3,5-trimethylcyclohexanone or 3,3,5- trimethylcyclohexanol, from which trimethyladipic acid is obtained by oxidation [78]. Trimethyladipic acid is used for the production of 2,2,4- trimethylhexamethylenediamine, which is a precursor for polyamides and polyurethanes. Addition of hydrogen cyanide to the olefinic double bond, followed by amination of the keto group in the presence of hydrogen, gives 3,5,5-trimethyl-3-aminomethylcyclohexylamine [2855-13-2], isophorone diamine. Reaction of the latter with phosgene gives isophorone diisocyanate [4098-71-9]. Both compounds are used in large quantities for the production of polymers, mainly polyurethanes. Worldwide production capacity for isophorone is ca. 50 000 t/a.
Definition
ChEBI: Isophorone is a cyclic ketone, the structure of which is that of cyclohex-2-en-1-one substituted by methyl groups at positions 3, 5 and 5. It has a role as a solvent and a plant metabolite. It is a cyclic ketone and an enone.
Preparation
Isophorone is produced on a multi-thousand ton scale by the aldol condensation of acetone using KOH. Diacetone alcohol, mesityl oxide, and 3-hydroxy-3,5,5-trimethylcyclohexan-1-one are intermediates. A side product is beta-isophorone, where the C=C group is not conjugated with the ketone.
Aroma threshold values
Detection: 0.20 ppm; aroma characteristics at 1.0%: cooling, woody, camphoraceous, slightly green and herbal
Taste threshold values
Taste characteristics at 30 ppm: sweet, green, waxy, pungent camphoreous, cooling minty
Synthesis Reference(s)
Synthetic Communications, 22, p. 1845, 1992 DOI: 10.1080/00397919208021315
Synthesis, p. 905, 1978 DOI: 10.1055/s-1978-24936
General Description
A clear colorless liquid, with a camphor-like odor. Less dense than water and insoluble in water. Boiling point 420°F. Flash point near 200°F. Contact irritates skin and eyes. Toxic by ingestion. Used as a solvent and in pesticides.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Ketones, such as Isophorone, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. Forms explosive peroxides
Health Hazard
Isophorone is an irritant, moderately toxic athigh concentrations, mutagenic and possiblycarcinogenic. Inhalation of its vapors cancause mild irritation of the eyes, nose, andthroat. Exposure to 840 ppm for 4 hours resulted in severe eye irritation in guineapigs. Its irritation effect on human eyes maybe felt at 25–40 ppm. At concentrationsabove 200 ppm, it may cause irritation ofthe throat, headache, nausea, dizziness, and afeeling of suffocation (ACGIH 1986). In rats,exposure to 1840 ppm for 4 hours was fatal.Ingestion of isophorone can cause narcosis,dermatitis, headache, and dizziness.
LD50 value, oral (rats): 2330 mg/kg
Isophorone is mutagenic and when fed torats orally, 258 g/kg for 2 years, it causedkidney tumor. Its carcinogenicity on humansis not reported.
Fire Hazard
Combustible.
Flammability and Explosibility
Not classified
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Industrial uses
Isophorone is an excellent solvent for cellulose esters, nitrocellulose, natural and synthetic resins. Isophorone has a very high aromatic hydrocarbon dilution ratio in nitrocellulose formulations: 5.7 for toluene and 5.1 for xylene. The excellent solvency of isophorone allows the preparation of 45% solids nitrocellulose solutions at room temperatures.
Biochem/physiol Actions
Taste at 30 ppm
Synthesis
Isophorone is an intermediate in the synthesis of 3,5-xylenol, 3,3,5-trimethylcyclohexanol
Carcinogenicity
In a 2 year bioassay in rats and mice (conducted by gavage on a 5- day/week treatment schedule) at dose levels of 0, 250, and 500 mg/kg/day isophorone, there was decreased survival of male rats and slight nephrotoxicity in female rats. There was no evidence of carcinogenicity in female rats or mice. In male rats, there was an increase in renal tumors in animals given either 250 or 500 mg/kg/day, and a low incidence of preputial gland tumors at 500 mg/kg. Other proliferative lesions in male rats included hyperplasia of the renal pelvis and tubular cell hyperplasia.
Environmental Fate
Biological. The pure culture Aspergillus niger biodegraded isophorone to 3,5,5-trimethyl-2-
cyclo-hexene-1,4-dione, 3,5,5-trimethylcyclohexane-1,4-dione, (S)-4-hydroxy-3,5,5-trimethyl-2-
cyclohex-1-one, and 3-hydroxymethyl-5,5-dimethyl-2-cyclohexen-1-one (Mikami et al., 1981).
Chemical/Physical. At an influent concentration of 1,000 mg/L, treatment with GAC resulted in
an effluent concentration of 34 mg/L. The adsorbability of the carbon used was 193 mg/g carbon
(Guisti et al., 1974).
Isophorone will not hydrolyze in water.
At influent concentrations of 10, 1.0, 0.1, and 0.001 mg/L, the GAC adsorption capacities were
78.3, 32.0, 13.1, and 5.4 mg/g, respectively (Dobbs and Cohen, 1980).
Purification Methods
Wash isophorone with aqueous 5% Na2CO3 and then distil it under reduced pressure immediately before use. Alternatively, it can be purified via the semicarbazone. [Erskine & Waight J Chem Soc 3425 1960, Beilstein 7 IV 165.]
Toxicity evaluation
The toxicological mechanisms of isophorone are not well characterized. Critical effects include irritation, narcosis, malaise, fatigue, and CNS depression. Isophorone may induce its neurological effects by interference with neuronal impulse transmissions via physical interaction with nerve membrane components. In animal models, isophorone may also act by inducing neuropathy, involving binding to globulin proteins, although this mechanism may not be relevant to humans. Lesions of the liver have been observed after overexposure in mouse models, although it is not clear whether isophorone elicited the lesions directly or by enhancing an age-related process. DNA-binding studies in mice have shown no significant covalent binding of isophorone or its metabolites to DNA from liver or kidney cells, supporting a potential nongenotoxic mechanism of toxicity.
Toxics Screening Level
The initial threshold screening level (ITSL) for isophorone is 280 μg/m3 (1-hour average).
Waste Disposal
Incineration.
Isophorone Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12809 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5868 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3005 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 | admin@hbsaisier.cn | China | 1015 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +8617736087130 | catherine@yjchem.com.cn | China | 994 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 +86-18633929156 | admin@hblongbang.com | China | 972 | 58 |
| Hebei Youfeidi Trading Co., LTD | +8615530197691 | admin@yfdchem.cn | China | 105 | 58 |
View Lastest Price from Isophorone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-25 | Isophorone
78-59-1
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd | |
 |
2025-04-25 | Isophorone
78-59-1
|
US $0.00-0.00 / kg | 1kg | 99% | 1000 | Hebei Junhua Import and Export Co., LTD | |
 |
2025-04-23 | Isophorone
78-59-1
|
US $1.00 / KG | 1KG | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd |
-

- Isophorone
78-59-1
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd
-

- Isophorone
78-59-1
- US $0.00-0.00 / kg
- 99%
- Hebei Junhua Import and Export Co., LTD
-

- Isophorone
78-59-1
- US $1.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
78-59-1(Isophorone)Related Search:
1of4