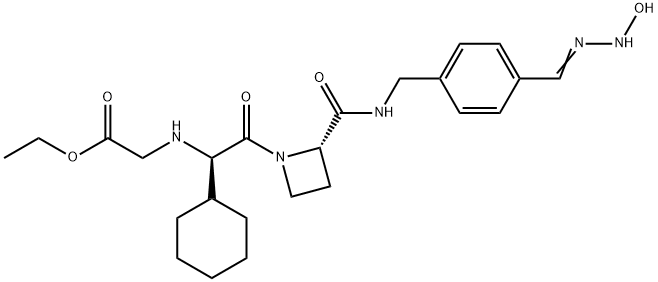
EXANTA
- CAS No.
- 192939-46-1
- Chemical Name:
- EXANTA
- Synonyms
- EXANTA;Exarta;CS-1834;H 376/95;Exanta (TN);XIMELEGATRAN;EXANTA/XIMELEGATRAN;Exanta (XiMelagatran);Ximelagatran [USAN:INN];Ximelagatran (H 376/95)
- CBNumber:
- CB7838374
- Molecular Formula:
- C24H35N5O5
- Molecular Weight:
- 473.57
- MDL Number:
- MFCD05861056
- MOL File:
- 192939-46-1.mol
- MSDS File:
- SDS
| Melting point | 65-68?C |
|---|---|
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO : 250 mg/mL (527.91 mM; Need ultrasonic)Methanol : 62.5 mg/mL (131.98 mM; Need ultrasonic) |
| pka | 6.87±0.69(Predicted) |
| form | powder |
| color | white to beige |
| Stability | Hygroscopic |
| FDA UNII | 49HFB70472 |
EXANTA price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1768 | Ximelagatran ≥98% (HPLC) | 192939-46-1 | 5MG | $174 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML1768 | Ximelagatran ≥98% (HPLC) | 192939-46-1 | 25MG | $698 | 2024-03-01 | Buy |
| Cayman Chemical | 16862 | Ximelagatran ≥98% | 192939-46-1 | 1mg | $35 | 2024-03-01 | Buy |
| Cayman Chemical | 16862 | Ximelagatran ≥98% | 192939-46-1 | 10 mg | $313 | 2024-03-01 | Buy |
| Cayman Chemical | 16862 | Ximelagatran ≥98% | 192939-46-1 | 500μg | $71 | 2023-01-06 | Buy |
EXANTA Chemical Properties,Uses,Production
Description
Ximelagatran, a prodrug of melagatran with improved oral bioavailability, is a direct thrombin inhibitor that was launched for the prevention of venous thromboembolic events (VTE) in elective hip or knee replacement surgery in Germany with several European countries following with approval for the same indication. A mutual recognition European filing was subsequently submitted for the prevention of stroke and other thromboembolic complications associated with atrial fibrillation (AF). While studies indicate that ximelagatran is as effective as traditional therapies for preventing strokes and recurring blood clots, the U.S. Food and Drug Administration has currently declined approval due to potential hepatotoxicity. Elevation of alanine aminotransferase (three times the upper limit of normal) has been observed in the first four months of therapy, but levels regress to normal upon discontinuation of the drug. Despite the questions surrounding the toxicological consequences of this elevated liver enzyme, ximelagatran remains an attractive alternative to the current antithrombotic therapies that utilize either the low molecular weight heparin (LMWH) or warfarin. Since LMWH is administered subcutaneously once or twice daily, the oral agent ximelagatran is preferable for patient compliance. In addition to the convenience of oral therapy, ximelagatran does not require the frequent laboratory monitoring and dosage adjustment that is necessary with warfarin treatment. A clinical study comparing the efficacy of a fixed dose (36 mg b.i.d.) of ximelagatran with adjusted dose warfarin for stroke prevention in patients with nonvalvular atrial fibrillation concluded that ximelagatran is not inferior to warfarin, and major bleeding occurred at rates similar to warfarin. The synthesis route to ximelagatran involves the coupling of the three major components, cyclohexylglycine, azetidine-2-carboxylic acid, and protected p-amidinobenzylamine, using solution-phase peptide chemistry. Subsequent alkylation of the N-terminus with ethyl bromoacetate, followed by deprotection of the amidine group and conversion to hydroxyamidine affords the double prodrug of melagatran. Delivery as ximelagatran provides reproducible oral bioavailability (18–25%), as measured by concentrations of the active metabolite melagatran formed by hydrolysis of the ethyl ester and dehydroxylation of the amidine. Melagatran reversibly binds to the arginine side pocket of both free and clot-bound thrombin (Ki=2 nM). Inhibition of thrombin ultimately blocks the conversion of fibrinogen to fibrin, the final step of the coagulation process. A linear relationship between ximelagatran dose and melagatran concentration exists with peak concentrations observed two to three hours post dose. Renal excretion is the primary route of elimination of melagatran (80%) with a half-life of 3–5 hours. Furthermore, the pharmacokinetics of ximelagatran is not influenced by the type of thromboembolic disease, obesity, ethnicity, gender, or age. In addition to the typical contraindications of current antithrombotic therapies, the increase in the liver enzyme alanine aminotransferase suggests that ximelagatran should not be used in patients with creatine clearance <30 mL/min. pending further study in this population. While ximelagatran does not appear to have any interactions with the cytochrome P-450 system, combination with aspirin has been shown to increase adverse bleeding.
Description
Ximelagatran is an ester prodrug of melagatran, a potent, direct, and reversible thrombin inhibitor (Ki = 1.2 nM). While melagatran has poor oral bioavailability, ximelagatran displays good bioavailability resulting, in part, from rapid absorption at the gastrointestinal tract, as well as rapid onset of action. Ximelagatran is converted to melagatran by reduction and hydrolysis at the liver and other tissues. It is used as an anticoagulant in a variety of situations, including thromboembolic disorders, stroke prevention in atrial fibrillation, and therapy in vein thrombosis.
Chemical Properties
Off-White Amorphous Solid
Originator
AstraZeneca (Germany)
Uses
An orally active direct thrombin inhibitor; prodrug of Melagatran. Antithrombotic.
Definition
ChEBI: A member of the class of azetidines that is melagatran in which the carboxylic acid group has been converted to the corresponding ethyl ester and in which the amidine group has been converted to the corresponding amidoxime. A prodrug for melagatran, ximela atran was the first orally available direct thrombin inhibitor to be brought to market as an anticoagulant, but was withdrawn in 2006 following reports of it causing liver damage.
brand name
Exanta (proposed) (AstraZeneca).
Biochem/physiol Actions
Ximelagatran is orally active, selective and potent direct thrombin inhibitor. Ximelagatran is a prodrug of thrombin inhibitor melagatran.
EXANTA Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9206 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | trendseenbio@gmail.com | China | 11681 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| Wuhan Jingkang en Biomedical Technology Co., Ltd | +8613720134139 | orders@jknbiochem.com | China | 5221 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131957 | 58 |
| Changsha Fuzhen Biotechnology Co.,LTD | 13823398779 18670069958 | 3062105966@qq.com | China | 3262 | 58 |
View Lastest Price from EXANTA manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Ximelagatran
192939-46-1
|
US $36.00-128.00 / mg | 98.09% | 10g | TargetMol Chemicals Inc. |
-

- Ximelagatran
192939-46-1
- US $36.00-128.00 / mg
- 98.09%
- TargetMol Chemicals Inc.