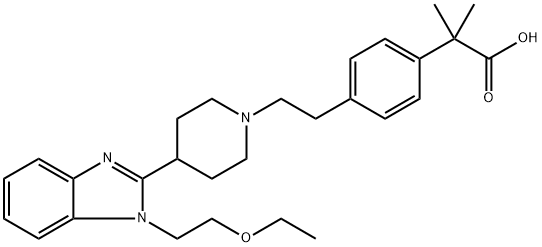
Bilastine
- CAS No.
- 202189-78-4
- Chemical Name:
- Bilastine
- Synonyms
- Bilastine;Bilastin;2-(4-(2-(4-(1-(2-ETHOXYETHYL)-1H-BENZO[D ]IMIDAZOL-2-YL) PIPERIDIN-1-YL)ETHY)PHEN YL)-2-METHYLPROPANOIC ACID;Bilaxten;Bilatex;Bilasten;Bilastene;Bilastine -IP/BP/;Bilastine (Form-I);Bilastine In-House
- CBNumber:
- CB81011081
- Molecular Formula:
- C28H37N3O3
- Molecular Weight:
- 463.62
- MDL Number:
- MFCD09837814
- MOL File:
- 202189-78-4.mol
| Melting point | 202 °C |
|---|---|
| Boiling point | 639.1±55.0 °C(Predicted) |
| Density | 1.16±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| Water Solubility | Insoluble in water |
| solubility | DMSO:49.3(Max Conc. mg/mL);106.34(Max Conc. mM) |
| pka | 4.40±0.10(Predicted) |
| form | powder to crystal |
| color | White to Light yellow |
| FDA UNII | PA1123N395 |
| ATC code | R06AX29 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P280-P305+P351+P338 | |||||||||
| HS Code | 2933.39.4100 | |||||||||
| NFPA 704 |
|
Bilastine price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | B5392 | Bilastine >98.0%(HPLC)(T) | 202189-78-4 | 250mg | $104 | 2024-03-01 | Buy |
| TCI Chemical | B5392 | Bilastine >98.0%(HPLC)(T) | 202189-78-4 | 1g | $286 | 2024-03-01 | Buy |
| Cayman Chemical | 28375 | Bilastine | 202189-78-4 | 25mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 28375 | Bilastine | 202189-78-4 | 50mg | $84 | 2024-03-01 | Buy |
| Cayman Chemical | 28375 | Bilastine | 202189-78-4 | 100mg | $155 | 2024-03-01 | Buy |
Bilastine Chemical Properties,Uses,Production
Description
Bilastine, a potent and selective histamine H1 receptor antagonist, was
approved in Europe in 2010 for the treatment of allergic rhinoconjunctivitis
(AR) and urticaria (hives or skin rash). The original synthesis of bilastine involves alkylation of 2-piperidinyl-1H-benzimidazole with a phenethyltosylate,
the para position of which is substituted with a dimethyloxazoline
moiety serving as a masked carboxylic acid group. Alkylation of the
benzimidazole nitrogen with 2-chloroethyl ethyl ether followed by
unmasking of the oxazoline moiety with sulfuric acid provided bilastine.
In two major clinical trials, bilastine was effective
at relieving allergic rhinitis as assessed by measuring the severity of nasal (obstruction, rhinorrhea, itching, sneezing) and nonnasal (ocular
itching, tearing, ocular redness, itching of ears, and/or palate) symptoms.
Description
Bilastine is a histamine H1 receptor antagonist (IC50 = 180 nM). It is selective for the histamine H1 receptor in a panel of 30 receptors in vitro at 100 μM. Bilastine prevents microvascular extravasation (ED50 = 185 μg/kg, i.v.), bronchospasm (ED50 = 4.6 μg/kg, i.v.), and systemic anaphylaxis (ED50 = 0.2 μg/kg, p.o.) induced by subcutaneous histamine in guinea pigs. It prevents anaphylaxis induced by subcutaneous administration of ovalbumin or dinitrophenylated human albumin (DNP) in sensitized rats when administered at doses of 7.6 and 6.0 mg/kg, respectively. Formulations containing bilastine have been used in the treatment of urticaria and allergic rhinitis.
Originator
FAES FARMA, S.A. (Spain)
Uses
Labelled Bilastine. It is a novel, nonsedating H1-antihistamine developed for symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria.
Uses
Bilastine is a novel, nonsedating H1-antihistamine developed for symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria.
Definition
ChEBI: Bilastine is a member of benzimidazoles.
brand name
Bilaxten
Clinical Use
Bilastine is a selective histamine H1 antagonist approved for the treatment of allergic rhinoconjunctivitis and urticaria (hives). This drug, which has proven to be well tolerated in toxicology profiling, 46 was discovered by the Spainsh firm FAES Farma and was approved by the European Union in 2010.
Synthesis
In 2011, Collier and
co-workers published a communication describing both the original
synthesis of bilastine and an improved route which was
amenable to gram-scale production. Collier?ˉs second generation
route, shown below, relies upon a convergent approach
involving the union of piperidinyl benzimidazole 49 with fully
functionalized phenethyl electrophile 48.Coupling the commercially
available bromophenyl acetate 44 with cyclic trioxatriborinane
45 under conventional Suzuki conditions furnished styrene
46 in good yield. Alternatively, this vinylation reaction was also
performed under Stille conditions with tributyl vinyl stannane in
83% yield. Hydroboration¨Coxidation of 46 delivered phenethyl
alcohol 47 which was then immediately mesylated under basic
conditions in toluene to produce adduct 48. This sulfonate was
then reacted with piperidine 49 (whose preparation is described
in Scheme 7) followed by saponification of the resulting ester 50
to arrive at bilastene (VI) in 26% overall yield from 44.
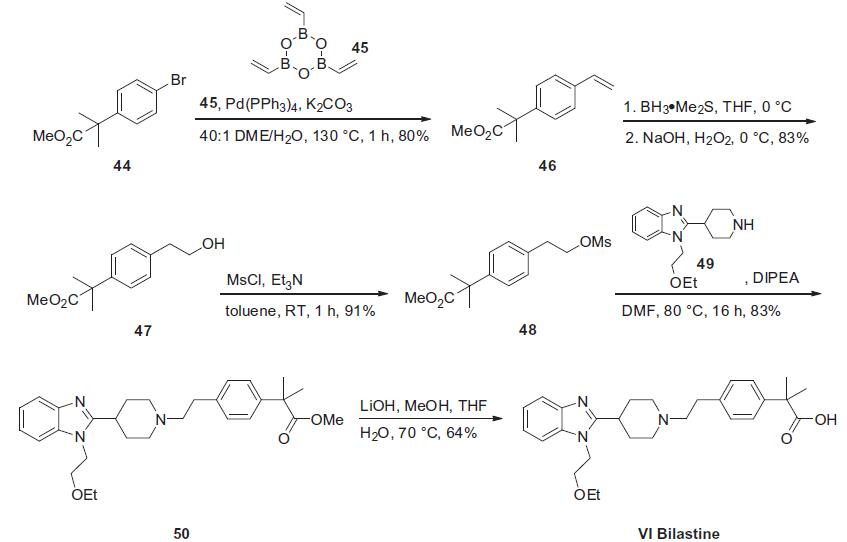
For the preparation of bilastine piperidine 49,
commercially available piperidine 51 was first protected as the Boc-carbamate 52 prior to alkylation of the benzimidazole nitrogen
atom with 1-chloro-2-ethoxyethane 53, providing compound
54. The Boc group of 54 was removed under acidic conditions to
give fragment 49. This sequence produced the desired piperidine
component in 86% overall yield from 51.
Drug interactions
Potentially hazardous interactions with other drugs
Antivirals: concentration possibly increased by
ritonavir.
Grapefruit juice: concentration of bilastine reduced.
Metabolism
Not significantly metabolised. Almost 95% of the administered dose was recovered in urine (28.3%) and faeces (66.5%) as unchanged bilastine
Bilastine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29882 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | stella@zetchem.com | China | 2136 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
View Lastest Price from Bilastine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | Bilastine
202189-78-4
|
US $0.00-0.00 / kg | 1kg | 99%, Single impurity<0.1 | 1 ton | Nanjing Fred Technology Co., Ltd | |
 |
2024-11-19 | Bilastine
202189-78-4
|
US $39.00-105.00 / mg | 99.61% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-03-26 | Bilastine
202189-78-4
|
US $3.00-0.20 / KG | 1KG | 98% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd |
202189-78-4(Bilastine)Related Search:
1of4