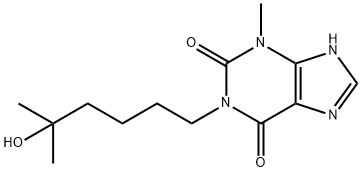
1-(5-hydroxy-5-methylhexyl)-3-methylxanthine
- CAS No.
- 107767-55-5
- Chemical Name:
- 1-(5-hydroxy-5-methylhexyl)-3-methylxanthine
- Synonyms
- HWA-138;1-(5-hydroxy-5-methylhexyl)-3-methylxanthine;3,7-Dihydro-1-(5-hydroxy-5-methylhexyl)-3-methyl-1H-purine-2,6-dione;1H-Purine-2,6-dione, 3,9-dihydro-1-(5-hydroxy-5-methylhexyl)-3-methyl-
- CBNumber:
- CB81318128
- Molecular Formula:
- C13H20N4O3
- Molecular Weight:
- 280.32
- MDL Number:
- MFCD00884660
- MOL File:
- 107767-55-5.mol
| Boiling point | 527.2±56.0 °C(Predicted) |
|---|---|
| Density | 1.270±0.06 g/cm3(Predicted) |
| pka | 8.62±0.70(Predicted) |
| FDA UNII | A3E4BQL3WG |
1-(5-hydroxy-5-methylhexyl)-3-methylxanthine Chemical Properties,Uses,Production
Originator
HWA-138 ,Hoechst-Roussel
Manufacturing Process
1-(5-Hydroxy-5-methylhexyl)-3-methylxanthine may be prepared next way:
1). 1-Chloro-5-hydroxy-5-methylhexane:
A solution of 67.3 g (0.5 mol) of 1-chloro-5-hexanone in 50 ml of anhydrous
ether is added dropwise to 44.9 g (0.6 mol) of methyl magnesium chloride in
the form of a 20% strength solution in tetrahydrofuran and 200 ml of dry
ether at 0° to 5°C, while stirring. The mixture is then subsequently stirred
initially at room temperature for one hour and then while boiling under reflux
for a further hour, the tertiary alkanolate formed is decomposed by addition of
50% strength aqueous ammonium chloride solution, the ether phase is
separated off and the aqueous phase is extracted by shaking with ether. The
combined ethereal extracts are washed in succession with aqueous sodium
bisulfite solution and sodium bicarbonate solution as well as a little water,
dried over sodium sulfate, filtered and concentrated in vacuo and the liquid
residue is subjected to fractional distillation under reduced pressure. Yield:
64.1 g (85.1% of theory), boiling point (20 mbar) 95-97°C, refractive index
nD25 =1.4489.
2). 7-Benzyl-3-methylxanthine:
20 g (0.5 mol) of sodium hydroxide dissolved in 200 ml of water are added to
a suspension of 83 g (0.5 mol) of 3-methylxanthine in 500 ml of methanol,
the mixture is stirred at 70°C for one hour, 85.5 g (0.5 mol) of benzyl
bromide are then added dropwise at the same temperature and the reaction
mixture is kept between 70°C and 80°C for 5 hours. It is then cooled and
filtered cold with suction, the product on the suction filter is washed with
water and dissolved in 1000 ml of 1 N sodium hydroxide solution under the
influence of heat, the solution is filtered and the pH is brought slowly to 9.5
with 4 N hydrochloric acid, while stirring. The crystals are filtered off from the
still warm solution, washed with water until free from chloride and dried in
vacuum. Yield: 81.7 g (63.8% of theory), melting point: 262-264°C.
3). 7-Benzyl-1-(5-hydroxy-5-methylhexyl)-3-methylxanthine:
A mixture of 20.5 g (0.08 mol) of 7-benzyl-3-methylxanthine, 12.4 g (0.09
mol) of potassium carbonate and 13.61 g (0.09 mol) of above 1-chloro-5-
hydroxy-5-methylhexane in 300 ml of dimethylformamide is heated at 110° to
120°C for 8 hours, while stirring, and is then filtered hot and the filtrate is
evaporated under reduced pressure. The residue is taken up in chloroform,
the mixture is washed first with 1 N sodium hydroxide solution and then with water until neutral and dried, the solvent is distilled off in vacuum and the
solid residue is recrystallized from ethyl acetate, with the addition of
petroleum ether Yield: 23.8 g (80.3% of theory), melting point: 109-111°C.
4). 1-(5-Hydroxy-5-methylhexyl)-3-methylxanthine:
14.8 g (0.04 mol) of the above mentioned 7-benzylxanthine are hydrogenated
in 200 ml of glacial acetic acid over 1.5 g of palladium (5%) on active
charcoal at 60°C under 3.5 bar in the course of 24 hours, while shaking. After
cooling, the mixture is blanketed with nitrogen, the catalyst is filtered off, the
filtrate is concentrated under reduced pressure and the solid residue is
recrystallized from ethyl acetate. Yield of albifylline: 9.6 g (85.6% of theory),
MP: 192-193°C.
Therapeutic Function
Vasodilator
1-(5-hydroxy-5-methylhexyl)-3-methylxanthine Preparation Products And Raw materials
Raw materials
Preparation Products
1-(5-hydroxy-5-methylhexyl)-3-methylxanthine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32161 | 58 |
| TargetMol Chemicals Inc. | +8613564774135 | zijue.cai@tsbiochem.com | United States | 19885 | 58 |
| Changzhou Bojia Biomedical Technology Co., Ltd. | 2122619822 | czbjpharma@126.com | China | 18478 | 58 |
| TargetMol Chemicals Inc. | 15002134094 | marketing@targetmol.cn | China | 19704 | 58 |
| Supplier | Advantage |
|---|---|
| TargetMol Chemicals Inc. | 58 |
| TargetMol Chemicals Inc. | 58 |
| Changzhou Bojia Biomedical Technology Co., Ltd. | 58 |
| TargetMol Chemicals Inc. | 58 |
View Lastest Price from 1-(5-hydroxy-5-methylhexyl)-3-methylxanthine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-28 | Albifylline
107767-55-5
|
US $1980.00-1520.00 / mg | 10g | TargetMol Chemicals Inc. |
-

- Albifylline
107767-55-5
- US $1980.00-1520.00 / mg
- TargetMol Chemicals Inc.