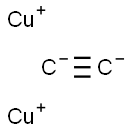COPPER(I) ACETYLIDE
- CAS No.
- 1117-94-8
- Chemical Name:
- COPPER(I) ACETYLIDE
- Synonyms
- COPPER(I)ACETYLIDE
- CBNumber:
- CB81423347
- Molecular Formula:
- Molecular Weight:
- 0
- MDL Number:
- MOL File:
- 1117-94-8.mol
COPPER(I) ACETYLIDE Chemical Properties,Uses,Production
Chemical Properties
amorphous red powder(s); unstable; oxidizes in air to Cu2O, carbon, and water, structure CuC≡CCu; can explode when subjected to shock or heated; obtained by reacting acetylene, HC≡CH, with soluble cuprous salt in water; used in detonators and other explosives [HAW93] [KIR78]
Physical properties
Red amorphous powder; explodes on heating; insoluble in water; soluble in acids.
Uses
Cuprous acetylide is used as a diagnostic testto identify the ≡CH unit; to purify acetylene;in the preparation of pure copper powder;and as a catalyst in the manufacture of 2-propyn-1-ol and acrylonitrile.
Preparation
Copper(I) acetylide is prepared by passing acetylene gas over an aqueous solution of ammoniacal copper saltHCCH + 2Cu(NH3)2OH → CuCCCu + 4NH3 + 2H2O.
Health Hazard
There are no toxicity data in the published literatureon cuprous acetylide. As with manycopper salts, inhalation of its dust can causeirritation of the respiratory tract and ulcerationof nasal septum.
Fire Hazard
Cuprous acetylide in the dry state is highly sensitive to shock, causing explosion on impact. It explodes in contact with acetylene after being warmed in air or oxygen for several hours (Mellor 1946). It forms a highly explosive mixture containing silver acetylide when mixed with silver nitrate. It ignites spontaneously with chlorine, bromine, and iodine vapors and with fine crystals of iodine. Reactions with dilute acids form acetylene, which is flammable and explosive in air. It explodes when heated above 100°C (212°F). It forms black copper(II)acetylide when exposed to air or oxygen.
Waste Disposal
Cuprous acetylide may be destroyed byslowly adding dilute hydrochloric acid toit in a three-necked flask under nitrogenflow. Acetylene formed is vented away withnitrogen.
COPPER(I) ACETYLIDE Preparation Products And Raw materials
Raw materials
Preparation Products
COPPER(I) ACETYLIDE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9020 | 58 |
| Supplier | Advantage |
|---|---|
| Shaanxi Didu New Materials Co. Ltd | 58 |