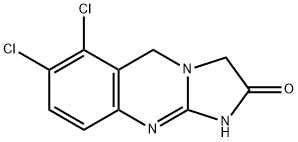
Anagrelide
- CAS No.
- 68475-42-3
- Chemical Name:
- Anagrelide
- Synonyms
- Anagrelida;ANAGRELIDE;Anagrelidum;Anagrelidebase;Anagrelide Monomer;Anagrelide USP/EP/BP;Anagrelide Impurity 25;Anagrelida|||Xagrid|||Anagrelidum;6,7-dichloro-1,5-dihydroiMidazo 2,1-b quinaz...;6,7-dichloro-1,5-dihydroimidazo[2,1-b]quinazolin-2(3H)one
- CBNumber:
- CB8246866
- Molecular Formula:
- C10H7Cl2N3O
- Molecular Weight:
- 256.09
- MDL Number:
- MFCD00866794
- MOL File:
- 68475-42-3.mol
- MSDS File:
- SDS
| Melting point | 280 °C |
|---|---|
| Density | 1.77±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| pka | pKa 2.87 (H2O t=25 I=0.025) (Uncertain);10(H2O t=25 I=0.025) (Uncertain) |
| Water Solubility | slightly soluble |
| CAS DataBase Reference | 68475-42-3(CAS DataBase Reference) |
| FDA UNII | K9X45X0051 |
| NCI Dictionary of Cancer Terms | anagrelide |
| ATC code | L01XX35 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazardous Substances Data | 68475-42-3(Hazardous Substances Data) |
Anagrelide price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | A637303 | Anagrelide | 68475-42-3 | 100mg | $500 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FA168205 | Anagrelide | 68475-42-3 | 50mg | $500 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0001487 | ANAGRELIDE 95.00% | 68475-42-3 | 1G | $811.65 | 2021-12-16 | Buy |
| AK Scientific | K139 | Anagrelide | 68475-42-3 | 5mg | $49 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FA168205 | Anagrelide | 68475-42-3 | 2mg | $60 | 2021-12-16 | Buy |
Anagrelide Chemical Properties,Uses,Production
Originator
Agrylin,Shire Pharmaceuticals
Uses
the treatment of primary thrombocytosis
Uses
Anagrelide; Potent PDE 3 inhibitorIt can be used to inhibit cancer cell invasion.
Definition
ChEBI: A 1,5-dihydroimidazo[2,1-]quinazoline having an oxo substituent at the 2-position and chloro substituents at the 6- and 7-positions.
Manufacturing Process
6,7-Dicloro-1,2,3,5-tetrahydroimidazo[2,1-b]quinazolin-2-one was produced
from 6-chloro-7-bromo-1,2,3,5-tetrahydroimidazo[2,1-b]quinozolin-2-one by
substitution the bromine an equimolar quantity chlorine.
6-Chloro-7-bromo-1,2,3,5-tetrahydroimidazo[2,1-b]quinazolin-2-one was
produced next way: to a solution of 1.30 g (8 mmole) of anhydrous ferric
chloride in 30 ml of nitromethane was added 1.30 g (5 mmole) of solid 6-
chloro-1,2,3,5-tetrahydroimidazo[2,1-b]quinazolin-2-one and 0.80 g (5
mmole) of bromine. The system was stoppered, warmed to 50°C in an oil
bath overnight, cooled to room temperature and the solvent removed in
vacuo. The resulting solid was suspended in water (50 ml), the mixture was
made basic (pH=10) with sodium bicarbonate and stirred at home
temperature for 20 min. The solid was filtered under suction, washed with
water, then isopropyl alcohol and dried yielding 1.19 g of 6-chloro-7-bromo-
1,2,3,5-tetrahydroimidazo[2,1-b]quinazolin-2-one (78% yield). Purification
was effected by formation of the hydrochloride salt (mp 275°C) from
acetonitrile.
6-Chloro-1,2,3,5,-tetrahydroimidazo[2,1-b]quinazolin-2-one was produced
from 6-chloro-2-nitrobenzylchloride, ethylglycine hydrochloride and cyanogen
bromide in 3 steps.
brand name
Agrylin (Shire).
Therapeutic Function
Platelet aggregation inhibitor
Clinical Use
Platelet-reducing agent
Enzyme inhibitor
This potent platelet aggregation inhibitor (FW = 256.09 g/mol; CAS 68475-42-3), also known as 6,7-dichloro-1,5-dihydroimidazo[2,1- b]quinazolin-2(3H)-one, BL-4162A, and Agrylin?, blocks the action of a variety of aggregating agents added platelet rich plasma, EC50 < 1 μg/mL, or 4 nM. Primary Mode of Action: Although the exact mechanism of its selective inhibition of megakaryocyte (MK) production of platelets remains uncertain, anagrelide is known to be a potent inhibitor of phosphodiesterase-II (IC50 = 36 nM) and lipoprotein-associated phospholipase A2 (or Lp-PLA2), the latter also known as platelet-activating factor acetylhydrolase (or PAF-AH). PDE-II hydrolyzes both cGMP and cAMP. Binding of cGMP to its regulatory GAF-B domain favors cAMP hydrolysis to 5’-AMP, thereby reducing cGMP hydrolysis to 5’-GMP. This property, which facilitates cross-regulation of the cAMP and cGMP pathways, suggests that a potent PDE-II inhibitor should potentiate the effects of cAMP and/or cGMP, the concentrations of which should increase in anagrelide-sensitive cells. Lp-PLA2 plays pivotal role in platelet maturation by specifically hydrolyzing Platelet-Activating Factor (PAF = acetyl-glyceryl-ether-phosphorylcholine) as well as other glycerophos-pholipids containing short, truncated, and/or oxidized fatty acyl groups at the sn-2 position of the glycerol backbone. At a final concentration of 100 ng/mL, anagrelide selectively blocks in vitro MK maturation, resulting in a 50% decrease in the total number of CD41a+ MKs. In humans, anagreline has the intriguing ability to promote as a species-specific platelet-lowering activity at dose levels lower than those required to inhibit platelet aggregation. Target(s): collagen- and immune complexinduced platelet aggregation and release; suppresses megakaryocytopoiesis by reducing the expression levels of the transcription GATA-1 and FOG-1 via a PDEIII-independent mechanism that is differentiation context-specific but does not involve inhibition of MPL-mediated early signal transduction events.
Drug interactions
Potentially hazardous interactions with other drugs
Aspirin: potential risks and benefits must first be
assessed, additive antiplatelet effect.
Cilostazol: avoid concomitant use.
Grapefruit juice: may reduce clearance of anagrelide.
Phosphodiesterase inhibitors: avoid with milrinone
and enoximone.
Metabolism
Anagrelide is primarily metabolised by CYP1A2; less than 1% is recovered in the urine as anagrelide. Two major urinary metabolites, 2-amino-5, 6-dichloro-3, 4-dihydroquinazoline and 3-hydroxy anagrelide (pharmacologically active) have been identified. The mean recovery of 2-amino-5, 6-dichloro-3, 4-dihydroquinazoline in urine is approximately 18-35% of the administered dose.
Anagrelide Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29810 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32161 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22787 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
| Wuhan Demeikai Biotechnology Co., Ltd | +8618942921723 | info@dmksw.xin | China | 717 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25356 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24727 | 58 |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | sales@chemhifuture.com | China | 3135 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38631 | 58 |
View Lastest Price from Anagrelide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Anagrelide
68475-42-3
|
US $67.00-221.00 / mg | 100% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Anagrelide
68475-42-3
|
US $67.00-221.00 / mg | 100% | 10g | TargetMol Chemicals Inc. | ||
 |
2021-08-05 | Anagrelide USP/EP/BP
68475-42-3
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited |
-

- Anagrelide
68475-42-3
- US $67.00-221.00 / mg
- 100%
- TargetMol Chemicals Inc.
-

- Anagrelide
68475-42-3
- US $67.00-221.00 / mg
- 100%
- TargetMol Chemicals Inc.
-

- Anagrelide USP/EP/BP
68475-42-3
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
68475-42-3(Anagrelide)Related Search:
1of4