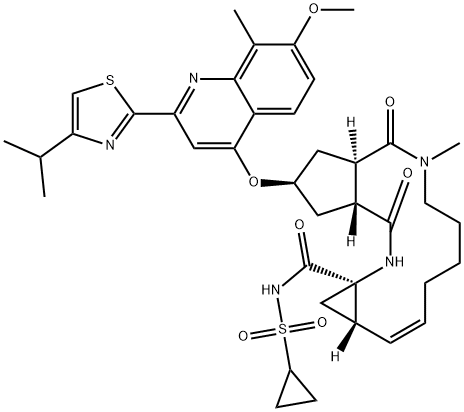
Simeprevir
- CAS No.
- 923604-59-5
- Chemical Name:
- Simeprevir
- Synonyms
- TMC435;CS-954;SiMeprevir;TMC 435350;siMeprevir/TMC435;Simeprevir-13C-d3;PubChem ID: 24873435;Simeprevir(TMC 435350);TMC435350 (Simeprevir);Simeprevir (sodium salt)
- CBNumber:
- CB82510099
- Molecular Formula:
- C38H47N5O7S2
- Molecular Weight:
- 749.94
- MDL Number:
- MFCD25563225
- MOL File:
- 923604-59-5.mol
- MSDS File:
- SDS
| Density | 1.38 |
|---|---|
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥18.75 mg/mL in DMSO |
| form | solid |
| pka | 4.47±0.40(Predicted) |
| color | White to off-white |
| FDA UNII | 9WS5RD66HZ |
| NCI Drug Dictionary | simeprevir |
| ATC code | J05AP05 |
| UNSPSC Code | 12352200 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
| Hazardous Substances Data | 923604-59-5(Hazardous Substances Data) |
Simeprevir price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 22144 | Simeprevir ≥98% | 923604-59-5 | 1mg | $35 | 2021-12-16 | Buy |
| Cayman Chemical | 22144 | Simeprevir ≥98% | 923604-59-5 | 5mg | $123 | 2021-12-16 | Buy |
| Cayman Chemical | 22144 | Simeprevir ≥98% | 923604-59-5 | 10mg | $228 | 2021-12-16 | Buy |
| Cayman Chemical | 22144 | Simeprevir ≥98% | 923604-59-5 | 25mg | $525 | 2021-12-16 | Buy |
| Usbiological | 466122 | Simeprevir | 923604-59-5 | 5mg | $425 | 2021-12-16 | Buy |
Simeprevir Chemical Properties,Uses,Production
Description
In September 2013, simeprevir (also known as TMC435) was approved in Japan (trade name Sovriad?) for the treatment of genotype 1 hepatitis C virus (HCV) infection in combination with pegylated interferon and ribavirin (PR). Simeprevir was approved for the same indication in November 2013 in the United States (trade name Olysio?) and Canada (trade name Galexos?). Simeprevir is the third HCV PI to receive approval and was discovered from an effort to optimize a novel series of cyclopentane-core macrocyclic HCV PIs. Unlike the earlier PIs, simeprevir does not rely on formation of a covalent intermediate to inhibit the enzyme, but instead gains binding affinity through a large P2 quinoline substituent that occupies an extended S2 subsite of HCV protease by induced fit. This pocket is not occupied by inhibitors such as telaprevir and boceprevir. Other key features of simeprevir are truncation of the P3 capping group (the N-methyl amide), use of an acylsulfonamide as an acid isostere, and incorporation of an isopropylthiazole group to give improved permeability. Simeprevir is a potent NS3/4A PI (Ki=0.36 nM), with antiviral activity in the HCV replicon assay (genotype 1b EC50=7.8 nM; genotype 1a EC50=28.4 nM). It is 25-fold less potent against HCV genotype 2, >1000 less potent for HCV genotype 3, but has 3-fold better potency for HCV genotype 4.
Originator
Tibotec and Medivir (Ireland and Sweden)
Uses
Simeprevir-d3 is labelled Simeprevir (S466500) which is a hepatitis C virus (NS3/4A) protease inhibitor. Simeprevir (S466500) is used for the cure and treatment of hepatitis C.
Definition
ChEBI: Simeprevir is an azamacrocycle and a lactam.
brand name
Sovriad
Clinical Use
HCV NS3/4A serine protease inhibitor:
Treatment of hepatitis C in combination with other
treatment
Synthesis
Commercial 2-methyl-3-methoxybenzoic acid (153) was treated
with diphenylphosphorylazide (DPPA) and triethylamine to
affect a Curtius rearrangement and the resulting isocyanate was
trapped with t-butanol to produce the Boc-protected aniline 154
in quantitative yield. Upon removal of the Boc protecting group
with TFA, the resulting aniline was reacted with boron trichloride
followed by the addition of acetonitrile and aluminum trichloride
to affect Friedel¨CCrafts acylation to give aminoacetophenone 155
in 40% yield. Acylation of the amino group with 4-isopropylthiazole-
2-carbonyl chloride (156) gave ketoamide 157 in 90% yield,
which was treated with potassium tert-butoxide in t-butanol at
100 ?? to furnish quinolinol 158 in 88% yield.
Use of a ring closing metathesis approach, enabling the synthesis
of the macrocyclic portion of the drug and ultimately simeprevir,
is described. Hydrogenation of commercial
trans-cyclopentanone-3,4-dicarboxylic acid (159) over Raney Ni in
the presence of triethylamine followed by cyclization to the
lactone using 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) and
N-methylmorpholine (NMM), and subsequent cinchonidine salt
formation gave lactone acid 160 in 26% yield over the 3 steps in
97% ee. Next, amide coupling with N-methylhexenylamine using
N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ), Fischer
esterification, and subsequent introduction of the quinolinol fragment
158 under Mitsunobu conditions using triphenylphosphine
(PPh3) and diisopropyl azodicarboxylate (DIAD) provided methyl
ester 161 in 65% overall yield for the three steps. Saponification
of the ester with lithium hydroxide followed by EEDQ-promoted
coupling to (1R,2S)-1-amino-2-vinyl-cyclopropane ethyl ester
(162) and Boc protection of the resulting amide gave the RCM
substrate, diene 163 in 95% yield for the two steps.
Macrocyclization of 163 using the second generation M2 catalyst
under dilute concentration in refluxing toluene followed
by acidic removal of the amide protecting group gave cycloalkene
ester 164 in high yield. Saponification of the ester, activation of the
resulting acid with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
(EDCI), and coupling with cyclopropylsulfonamide led to
simeprevir (XXI) in high overall yield.
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: possible increased risk of
bradycardia with amiodarone.
Antibacterials: concentration possibly increased by
clarithromycin - avoid; concentration of both drugs
increased with erythromycin - avoid; concentration
reduced by rifampicin and possibly rifabutin - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, oxcarbazepine,
phenobarbital, phenytoin and primidone - avoid.
Antifungals: concentration possibly increased by
fluconazole, itraconazole, ketoconazole, posaconazole
and voriconazole - avoid.
Antivirals: concentration of both drugs increased
with darunavir - avoid; concentration reduced
by efavirenz; avoid with etravirine; concentration
possibly reduced by nevirapine - avoid;
concentration increased by ritonavir - avoid.
Ciclosporin: avoid concomitant use, increased
simeprevir concentration.
Cobicistat: concentration possibly increased by
cobicistat - avoid.
Metabolism
Hepatically metabolised. In vitro experiments with human
liver microsomes indicated that simeprevir primarily
undergoes oxidative metabolism by the hepatic CYP3A4
system.
Elimination of simeprevir occurs via biliary excretion.
Following a single oral administration of 200 mg
[14C]-simeprevir to healthy subjects, on average 91% of
the total radioactivity was recovered in faeces. Unchanged
simeprevir in faeces accounted for on average 31% of the
administered dose. Renal clearance plays an insignificant
role in its elimination.
Simeprevir Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12814 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32159 | 58 |
View Lastest Price from Simeprevir manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-03-21 | Simeprevir Tmc 435350
923604-59-5
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd | |
 |
2024-11-19 | Simeprevir
923604-59-5
|
US $30.00-61.00 / mg | 99.77% | 10g | TargetMol Chemicals Inc. | ||
 |
2021-07-13 | Simeprevir
923604-59-5
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Simeprevir Tmc 435350
923604-59-5
- US $0.00 / KG
- 99%
- Hebei Mujin Biotechnology Co.,Ltd
-

- Simeprevir
923604-59-5
- US $30.00-61.00 / mg
- 99.77%
- TargetMol Chemicals Inc.
-

- Simeprevir
923604-59-5
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd