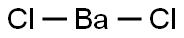Barium chloride
- CAS No.
- 10361-37-2
- Chemical Name:
- Barium chloride
- Synonyms
- BaCl2;Barium Chloride Solution, 10% w/w, neutralized;ba0108e;Ba 0108E;sba0108e;SBA 0108E;NCI-C61074;Dichlorobarium;BARIUM CHLORIDE;bariumdichloride
- CBNumber:
- CB8398703
- Molecular Formula:
- BaCl2
Lewis structure

- Molecular Weight:
- 208.23
- MDL Number:
- MFCD00003445
- MOL File:
- 10361-37-2.mol
- MSDS File:
- SDS
| Melting point | 963 °C (lit.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 1560°C | ||||||||||||||
| Density | 3.856 g/mL at 25 °C (lit.) | ||||||||||||||
| bulk density | 1350kg/m3 | ||||||||||||||
| storage temp. | 2-8°C | ||||||||||||||
| solubility | H2O: soluble | ||||||||||||||
| form | beads | ||||||||||||||
| color | White | ||||||||||||||
| Specific Gravity | 3.9 | ||||||||||||||
| PH | 5-8 (50g/l, H2O, 20℃) | ||||||||||||||
| Flame Color | Pale Green | ||||||||||||||
| Water Solubility | Soluble in water and methanol. Insoluble in acids, ethanol, acetone and ethyl acetate. Slightly soluble in nitric acid and hydrochloric acid. | ||||||||||||||
| Sensitive | Hygroscopic | ||||||||||||||
| Crystal Structure | PbCl2 type | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Merck | 14,971 | ||||||||||||||
| Space group | Pnma | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits |
ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
||||||||||||||
| Dielectric constant | 9.4(0.0℃) | ||||||||||||||
| Stability | Stable. | ||||||||||||||
| CAS DataBase Reference | 10361-37-2(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 1-4 | ||||||||||||||
| FDA UNII | 0VK51DA1T2 | ||||||||||||||
| NIST Chemistry Reference | Barium dichloride(10361-37-2) | ||||||||||||||
| EPA Substance Registry System | Barium chloride (10361-37-2) | ||||||||||||||
| UNSPSC Code | 12352305 | ||||||||||||||
| NACRES | NB.21 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H319-H332 | |||||||||
| Precautionary statements | P261-P264-P270-P301+P310-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | T,Xi,Xn | |||||||||
| Risk Statements | 22-25-20-36/37/38-36/38-36 | |||||||||
| Safety Statements | 45-36-26-36/37/39 | |||||||||
| RIDADR | UN 3264 8/PG 3 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.5 mg/m3 [*Note: The REL also applies to other soluble barium compounds (as Ba) except Barium sulfate.] | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | CQ8750000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2827 39 85 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| Hazardous Substances Data | 10361-37-2(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: 118 mg/kg | |||||||||
| NFPA 704 |
|
Barium chloride price More Price(50)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 202738 | Barium chloride 99.999% trace metals basis | 10361-37-2 | 5g | $48.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 202738 | Barium chloride 99.999% trace metals basis | 10361-37-2 | 25g | $130 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.60324 | Barium chloride solution c(BaCl2) = 0.1 mol; standardised against Zinc (CRM), Titripur? | 10361-37-2 | 1L | $85 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.09962 | Barium chloride solution for 1000 ml, c(BaCl?) = 0.05 mol/l (0.1 N) Titrisol? | 10361-37-2 | 1AMP | $46.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.01716 | Barium chloride 99.995 Suprapur? | 10361-37-2 | 50g | $358 | 2024-03-01 | Buy |
Barium chloride Chemical Properties,Uses,Production
Soluble barium salt
Barium chloride is one of the most important soluble barium salts, it is also known as "salting barium", the formula is BaCl2, it has colorless monoclinic and colorless cubic two crystals, monoclinic crystal turns into cubic crystal at 962℃. At room temperature it is white lustrous monoclinic crystal, odorless, bitter and salty. It is soluble in water, insoluble in acetone, ethanol and ether, slightly soluble in acid, sulfuric acid. Crystallization from aqueous solution of barium chloride often contains two crystal water. When heated to 113℃, it loses crystal water and becomes anhydrous barium chloride, it is white powder.

Barium chloride can be used for identification and separation of SO42-ions, purify the brine water, mainly used for the manufacture of barium salts and pigments, it can also be used as hard water softener, wool and leather industry mordant, pesticides for controlling plant pests. Preparation of industrial barium chloride is mainly used barite as material which containing high components of barium sulfate barite, coal and calcium chloride is mixed, and calcined to get barium chloride, reaction is as follows:
BaSO4 + 4C + CaCl2 → BaCl2 + CaS + 4CO ↑.
Chemical properties
It is colorless monoclinic crystal. (Α type) it is soluble in water, slightly soluble in hydrochloric acid, nitric acid, very slightly soluble in alcohol.
Uses
(1) Barium chloride is mainly used for heat treatment of metals, barium salt manufacturing, electronic instruments, and used as water softener.
(2) It can be used as dehydrating agent and analysis reagents, it is used for machining heat treatment Jing, see other barium chloride dihydrate.
(3) Calibration instruments and devices, evaluation methodology, quality assurance/quality control.
Hazardous characteristics
Barium chloride is noncombustible. It is highly toxic. When contacts boron trifluoride, violent reaction can occur. Swallowed or inhaled can cause poisoning, it is mainly through the respiratory tract and digestive tract to invade the human body, it will cause drooling and burning esophagus, stomach pain, cramps, nausea, vomiting, diarrhea, high blood pressure, no law firm pulse, cramps, a lot of cold sweat, weak muscle strength, gait, vision and speech problems, difficulty breathing, dizziness, tinnitus, consciousness usually clear. In severe cases, it can cause sudden death. Barium ions can cause muscle stimulant, then gradually transforms into paralysis. Rat oral LD50150mg/kg, mouse peritoneal LD5054mg/kg, rats are intravenously LD5020mg/kg, orally in dog LD5090mg/kg.
Production method
Barium chloride dihydrate is heated to above 150℃ by dehydration to obtain anhydrous barium chloride products. its
BaCl2 • 2H2O [△] → BaCl2 + 2H2O
Description
Barium dichloride is a white solid, odorless, hygroscopic chemical substance. Barium dichloride is used in the manufacture of pigments, in the manufacture of other barium salts and in fireworks to give a bright green color. It is one of the most common watersoluble salts of barium. Like other barium salts, it is toxic and imparts a yellow-green coloration to a flame. Barium chloride has wide application in the laboratory.
Chemical Properties
Barium chloride,BaCI2, is a colorless toxic salt with a melting point of 963°C. It is soluble in water. Barium chloride is used in metal surface treatment and as a rat poison.
Physical properties
Barium chloride has the formula, BaCl2 and is an ionic chemical compound. It is one of the most important water-soluble salts of barium-containing compounds. Like other barium salts, it is toxic and imparts a yellow-green coloration to a flame. It is also hygroscopic. Barium chloride was the by-product of the discovery of radium by Madame Curie (1898).When refining radium, the final separation resulted in barium chloride and radium chloride. BaCl2 crystallizes in both the cubic “fluorite” and “lead chloride” crystal structures, both of which accommodate the preference of the large Ba2+ ion for coordination numbers greater than six.
Uses
Barium chloride (BaCl2) is used in the manufacture of paint pigments and dyeing textiles and as an additive in oils. It is also used as a water softener.
Uses
Barium chloride is used in manufacture of pigments, fire works, other barium salts, fireworks , hardening of steel, heat treatment salts, purification of brine solution in caustic chlorine plants, lubrication oil additive, textile dye, pigments, white leather, aluminum refining, boiling water treatment and porcelain enamels for sheet steel. It is also used to remove sulfate ion in some electrolytic plants, caustic soda, magnesium metal or sodium metal. It is also used as a component in a flux used to prevent oxidation of molten magnesium.
Definition
ChEBI: The inorganic dichloride salt of barium.
Preparation
Barium chloride can be prepared from barium
hydroxide or barium carbonate, the latter being found
naturally as the mineral “Witherite”. These basic salts
react to give hydrated barium chloride. On an industrial
scale, it is prepared via a two-step process from the
mineral “Baryte”:
BaSO4+4C→BaS+4CO (gas)
This first step requires high temperatures. The second
step requires fusion of the reactants:
BaS+ CaCl2→BaCl2+CaS
The BaCl2 is then be leached out from the mixture
with water. From water solutions of barium chloride,
the dihydrate can then be crystallized as white crystals,
BaCl2·2H2O, which are colorless, translucent rhomboidal
tablets or lamellae. The dihydrate is stable in
the air at room temperature, but loses one-half of its
water above 55°C(131F), and becomes anhydrous at
121°C (250 F).
General Description
Any of a variety of substances that contain barium. Most are whitish colored crystalline solids. They tend to be soluble in water and denser than water. They may be toxic by inhalation or possibly skin absorption. They are often used to make other chemicals.
Air & Water Reactions
Water soluble.
Reactivity Profile
BARIUM CHLORIDE may react violently with BrF3 and 2-furan percarboxylic acid in its anhydrous form.
Hazard
Ingestion of 0.8 g may be fatal.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
reaction suitability
reaction type: Redox Reactions
Industrial uses
Barium chloride (BaCl2·2H2O) is a colorless, white powder highly soluble in water (25% at 10 °C). It is quite a toxic reagent. Barium chloride is used during borite flotation as an activator. Barium chloride also has a depressing effect on fluorite and cassiterite.
Safety Profile
A poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Inhalation absorption of barium chloride equals 60-80%; oral absorption equals 10-30%. Experimental reproductive effects. Mutation data reported. See also BARIUM COMPOUNDS (soluble). When heated to decomposition it emits toxic fumes of Cl-.
Barium chloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 | admin@zhanyaobio.com | China | 2123 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12814 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5870 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| Dalian Richfortune Chemicals Co., Ltd | 0411-84820922 8613904096939 | sales@richfortunechem.com | China | 304 | 57 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2957 | 58 |
Related articles
- Why does Barium Chloride burn green?
- When burned, barium chloride produces a bright green color in flames. It is also known to be hygroscopic, meaning it absorbs m....
- Jan 25,2024
- Property, preparation and application of barium chloride
- Barium dichloride is a white solid, odorless, hygroscopic chemical substance. Barium dichloride is used in the manufacture of ....
- Jul 7,2022
View Lastest Price from Barium chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-18 | Barium chloride
10361-37-2
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-03-21 | Barium chloride
10361-37-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd | |
 |
2025-03-07 | Barium Chloride Anhydrous;BaCl2
10361-37-2
|
US $0.80 / Kg/Bag | 20T | 99%;98% | 2000tons/month | Hebei Yanxi Chemical Co., Ltd.
|
-

- Barium chloride
10361-37-2
- US $10.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Barium chloride
10361-37-2
- US $0.00 / KG
- 99%
- Hebei Mujin Biotechnology Co.,Ltd
-

- Barium Chloride Anhydrous;BaCl2
10361-37-2
- US $0.80 / Kg/Bag
- 99%;98%
- Hebei Yanxi Chemical Co., Ltd.