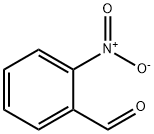
2-Nitrobenzaldehyde
- CAS No.
- 552-89-6
- Chemical Name:
- 2-Nitrobenzaldehyde
- Synonyms
- 2-NBA;2-nitro-benzaldehyd;O-NITROBENZALDEHYDE;2-Nitobenzaldehyde;1-ForMyl-2-nitrobenzene;2-Nitrobenzenecarboxaldehyde;NSC 5713;AKOS 93864;2-NitrobenzaL;AKOS BBS-00003153
- CBNumber:
- CB8414184
- Molecular Formula:
- C7H5NO3
- Molecular Weight:
- 151.12
- MDL Number:
- MFCD00007132
- MOL File:
- 552-89-6.mol
- MSDS File:
- SDS
| Melting point | 42-44 °C(lit.) |
|---|---|
| Boiling point | 153 °C23 mm Hg(lit.) |
| bulk density | 350kg/m3 |
| Density | 1,284 g/cm3 |
| vapor density | 5.2 (vs air) |
| refractive index | 1.5800 (estimate) |
| Flash point | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Crystalline Powder, Needles and/or Chunks |
| color | Yellow |
| Water Solubility | Insoluble in water. |
| Sensitive | Air Sensitive |
| Merck | 14,6586 |
| BRN | 742624 |
| LogP | 1.74 at 25℃ |
| CAS DataBase Reference | 552-89-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 48B18Q9B8E |
| NIST Chemistry Reference | Benzaldehyde, 2-nitro-(552-89-6) |
| EPA Substance Registry System | Benzaldehyde, 2-nitro- (552-89-6) |
| UNSPSC Code | 12352114 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335-H412 | |||||||||
| Precautionary statements | P273-P301+P312+P330-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38-68 | |||||||||
| Safety Statements | 26-24/25-36/37/39-36 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | CU7300000 | |||||||||
| F | 8 | |||||||||
| Autoignition Temperature | 200 °C (Lit.) | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29130000 | |||||||||
| NFPA 704 |
|
2-Nitrobenzaldehyde price More Price(42)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22293 | 2-Nitrobenzaldehyde for synthesis | 552-89-6 | 1G | $39.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22293 | 2-Nitrobenzaldehyde for synthesis | 552-89-6 | 25G | $69.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22293 | 2-Nitrobenzaldehyde for synthesis | 552-89-6 | 100G | $207 | 2024-03-01 | Buy |
| TCI Chemical | N0130 | 2-Nitrobenzaldehyde >99.0%(GC) | 552-89-6 | 25g | $53 | 2024-03-01 | Buy |
| TCI Chemical | N0130 | 2-Nitrobenzaldehyde >99.0%(GC) | 552-89-6 | 100g | $154 | 2024-03-01 | Buy |
2-Nitrobenzaldehyde Chemical Properties,Uses,Production
Chemical Properties
yellow or bright yellow needle-like crystals. It can volatilize with water vapor and has the fragrance of benzaldehyde. Soluble in ethanol, ether, benzene, slightly soluble in water, flash point>110℃, reacts violently with pyrrole.
Uses
2-Nitrobenzaldehyde is a benzaldhyde with a nitro group substituted in the ortho position. 2-Nitrobenzaldehyde is used in the preparation of dyes and colorants such as Indigo carmine. 2-Nitrobenzaldehyde gas been shown to be a useful photoremovable protecting group as well as in the preparation of more effective ones such as o-Nitrophenylethylene glycol.
Definition
ChEBI: 2-nitrobenzaldehyde is benzaldehyde substituted at the ortho-position with a nitro group. It is a C-nitro compound and a member of benzaldehydes.
Application
2-Nitrobenzaldehyde can react with chitosan to form 2-nitrobenzyl-chitosan, which is soluble in trifluoroacetic acid and can also be electrospun into nanofiber matrices. On photolysis, the nitrobenzyl group is cleaved to form neat chitosan nanofiber matrices. The same principle has been applied for the preparation of gelatin nanofiber matrices. The condensation of o-NBA with 2-aminobenzothiazole forms o-nitrobenzylidene 2-aminobenzothiazole, a Schiff′s base that can react with metal ions to form complexes.
Preparation
2-Nitrobenzaldehyde is synthesized from 2-Nitrotoluene by nitration and oxidation.
Synthesis of 2-Nitrobenzaldehyde from 2-Nitrotoluene
Synthesis Reference(s)
Synthetic Communications, 19, p. 3385, 1989 DOI: 10.1080/00397918908052745
General Description
The 2-Nitrobenzyl group of 2-nitrobenzaldehyde is photolabile that can be cleaved when exposed to UV-light.
Synthesis
50 g (0.24 mol) of 2-nitrophenylpyruvic acid of melting point 115° C are introduced into 500 ml of aqueous sodium carbonate until a clear solution is obtained. After the addition of 350 ml of toluene, the mixture is cooled to 0° C, and 40 g of solid potassium permanganate is then added in portions at 0°- 3° C. After one hour at 0°- 3° C, 95 ml of 50% strength sulphuric acid are added dropwise. The temperature is not allowed to rise above 30° C. The reaction mixture is filtered, and the toluene phase is separated from the filtrate. The filter residue is washed with toluene, and the combined toluene phases are extracted with 15% strength sodium carbonate solution and with water. The toluene phase is then dried with sodium sulfate and concentrated in a vacuo. The remaining residue consists of 19.7 g (54.7% of theory) of 2-nitrobenzaldehyde, which crystallizes on cooling.
Purification Methods
Crystallise the aldehyde from toluene (2-2.5mL/g) by addition of 7mL pet ether (b 40-60o) for 1mL of solution. It can also be distilled under reduced pressures. [Beilstein 7 IV 584.]
2-Nitrobenzaldehyde Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Changzhou AniKare Pharmatech Co., Ltd. | +86-0519-8359-8696 +8618018249389 | sales@anikare.com | China | 9875 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12809 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 | allison@yan-xi.com | China | 5854 | 58 |
| RongNa Biotechnology Co.,Ltd | +86-86-13583358881 +8618560316533 | Brad@rongnabiotech.com | China | 3084 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5868 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | mina@chuanghaibio.com | China | 18137 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8615965530500 | nickzhang@hangyubiotech.com | China | 10983 | 58 |
| Apeloa production Co.,Limited | +8619933239880 | admin@apcl.com.cn | China | 861 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
Related articles
- 2-Nitrobenzaldehyde: Key Properties and Applications in Analytical Chemistry
- 2-Nitrobenzaldehyde is a yellow aromatic aldehyde used as an antioxidant and actinometer, crucial for assessing UV light doses....
- Sep 24,2024
View Lastest Price from 2-Nitrobenzaldehyde manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-25 | 2-Nitrobenzaldehyde
552-89-6
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-04-25 | 2-Nitrobenzaldehyde
552-89-6
|
US $1.00 / g | 1g | 99% | 100kg | RongNa Biotechnology Co.,Ltd | |
 |
2025-04-23 | 2-Nitrobenzaldehyde
552-89-6
|
US $10.00 / KG | 1KG | 99% | 5tons | Hebei Chuanghai Biotechnology Co., Ltd |
-

- 2-Nitrobenzaldehyde
552-89-6
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- 2-Nitrobenzaldehyde
552-89-6
- US $1.00 / g
- 99%
- RongNa Biotechnology Co.,Ltd
-

- 2-Nitrobenzaldehyde
552-89-6
- US $10.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
552-89-6(2-Nitrobenzaldehyde)Related Search:
1of4