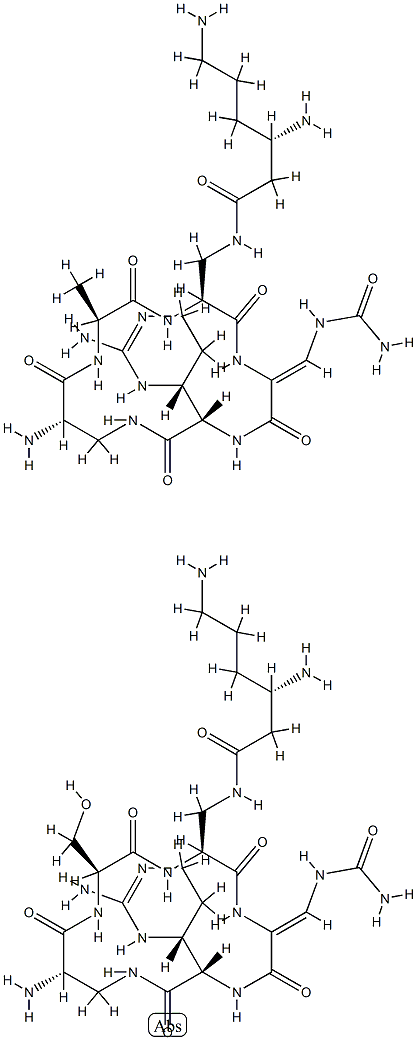
CAPREOMYCIN
- CAS No.
- 11003-38-6
- Chemical Name:
- CAPREOMYCIN
- Synonyms
- capastat;capromycin;capostatin;Tubermycin;kapreomycin;CAPREOMYCIN;CAPREOMYCINE;Capreomycin free base;CAPREOMYCIN USP/EP/BP;CaproMycin, Caprolin, Capastat, Capostatin, L 29275
- CBNumber:
- CB8471646
- Molecular Formula:
- C50H88N28O15
- Molecular Weight:
- 1321.41232
- MDL Number:
- MFCD01770630
- MOL File:
- 11003-38-6.mol
| pka | pKa in 66% aq DMF: 6.2, 8.2, 10.1, 13.3(at 25℃) |
|---|---|
| EWG's Food Scores | 1 |
| FDA UNII | 232HYX66HC |
| ATC code | J04AB30 |
SAFETY
Risk and Safety Statements
| Hazardous Substances Data | 11003-38-6(Hazardous Substances Data) |
|---|
CAPREOMYCIN Chemical Properties,Uses,Production
Description
Capreomycin is a kind of antibiotics for the treatment of tuberculosis. It should be noted that it is for second line treatment, being used for targeting active drug resistant tuberculosis. It is mainly used for the treatment of mycobacterium tuberculosis. It is a cyclic peptide antibiotic similar to viomycin, being produced by Streptomyces capreolus. It belongs to the aminoglycoside family of antibiotics. The exact mechanism of action of capreomycin is largely unknown. It is indicated that it blocks the protein synthesis of mycobacterium tuberculosis through binding to the 70S ribosome, and also inducing abnormal protein synthesis in bacteria.
References
https://www.drugbank.ca/drugs/DB00314
https://en.wikipedia.org/wiki/Capreomycin
Description
Capreomycin is a semisynthetic antibiotic (34.1.21) that is isolated from
the cultural fluid of Streptomyces capreolus, and it is a complex of a minimum of four
microbiologically active ingredients that have only partially been characterized.
Capreomycin has a pronounced suppressive effect against Mycobacterium tuberculosis
and Mycobacterium bovis. Most strains of Mycobacterium kansasii are also sensitive to
kanamycin, while other, nontuberculous strains are not sensitive to it. It is often used upon
necessity of using parenternal therapy through deep intramuscular injections.
Capreomycin is less toxic than kanamycin and has somewhat more of a bacteriostatic
effect. Synonyms of this drug are capromycin, capastat, ogostal, and others.
Originator
Capastat,Dista
Uses
Capreomycin is a complex of cyclic pentopeptides isolated from Streptomyces capreolus, first reported in 1962. The complex has two major components, IA and IB, with an exocyclic lysine residue and two minor delysinyl components, IIA and IIB. Capreomycin is a potent antibiotic with activity against mycobateria, Gram positive and Gram negative organisms. Capreomycin acts by binding to the 23S ribosomal subunit, disrupting protein synthesis.
Indications
Capreomycin (Capastat) is a polypeptide antibiotic derived from Streptomyces capreolus. It is bacteriostatic against most strains of M. tuberculosis, including the MDR strain. In addition, it is active against M. kansasii, M. avium, and in high concentrations, some grampositive and gram-negative bacteria. Like other antitubercular drugs, resistance to capreomycin occurs rapidly if the drug is used alone.There is no cross-resistance between streptomycin and capreomycin, but some isolates resistant to capreomycin are resistant to viomycin.
Manufacturing Process
Preparation of Capreomycin
A culture of NRRL 2773 is produced by growing the organism on a nutrient
agar slant having the following composition: Tomato paste 20 g, Pre-cooked
oatmeal 20 g, Agar 15 g and tap water up to 1 L. The slant is inoculated for
10 days at about 30°C. The culture growth on the slant is covered with 6 ml
of nutrient broth, and the slant is scraped gently to remove the organisms to
provide an aqueous suspension.
Employing aseptic techniques, the inocolum obtained is used to inoculate a 2
L Erlenmeyer flask containing a 500 ml portion of a sterilized vegetative
culture medium having the following composition: Soluble starch 10 g,
Peptones 5 g, Beef extract 5 g, Sodium chloride 5 g, Yeast extract 2.5 g and
tap water 1100 ml.
The incubation is carried out at 28°C for 48 hours; during which time the
flasks are shaken at the rate of 250 cycles per minute on a rotary shaker
having a 1-inch stroke. To produce a larger quantity of vegetative inocolum,
500 ml of the vegetative inocolum is added aseptically to a stainless steel
350-gallon fermentation tank containing 250 gallons of sterile medium having
following composition (weight/volume): Glucose 1.5%, Yeast 1.5%, Antifoam
("Polyglycol No 2000" sold by Dow Chemical Co) 0.02%.
The inoculum is allowed to grow for 22 hours at 30°C. Throughout the growth
period, the medium is aerated with sterile air at the rate of 17 cubic feet per
minute and is agitated with 16-inch impeller rotating at 160 revolution per
minute.
To a 1700-gallon stainless steel fermentor are added 1100 gallons of a
medium having following composition (weight/volume): Glucose 2.5%,
Molasses 1.0%, Peptones 4.0%, Calcium carbonate 0.2%, Hydrolyzed casein
0.6%, Antifoam ("Polyglycol No 2000" sold by Dow Chemical Co) 0.005%.
The medium after sterilization is inoculated with 100 gallons of the inoculum
grown in the fermentation tank. The fermentation is carried on at 30°C for 5
days. The foam is controlled by the addition, when needed, of "Larex No 1"
(an antifoam product sold by Swift and Co.). Throughout the fermentation,
the medium is aerated with sterile air at the rate of 17 cubic feet per minute
and is agitated with 22-inch impeller rotating at 140 revolution per minute. At
the end of the fermentation, 240 pounds of "Dicalite 476" (a perlite filler
product sold by Great Lakes Carbon Corporation) are added 1000 gallons or
the antibiotic broth, and the mixture is stirred and filtered. The filter cake is
washed with tap water and the filtrates are combined to provide a total
volume of 1000 gallons. To 500 gallons of the combined liquids are added 132
pounds of "Darco G-60". The mixture is filtered, and the filtrate is discarded.
The carbon filter cake is washed with 200 L of tap water, the wash water
being discarded.
The washed carbon cake on which the capreomycin is adsorbed is washed
with 200 L of 0.05 N aqueous hydrochloric acid. The acid wash is discarded.
The washed carbon cake is eluted during a one-hour period with 400 L of an
aqueous acetone containing 1.65 L of 11.7 N hydrochloric acid and 80 L of
acetone. The filter cake is further eluted by washing the cake with 200 L of an
aqueous acetone containing 825 ml of 11.7 N hydrochloric acid and 40 L of
acetone during a 15-minute period. The combined eluates, having a total
volume 575 L, are concentrated in vacuo to 52.5 L. The concentrate is added with stirring to 525 L of acetone and the acetone mixture is permitted to
stand overnight at room temperature, during which time an oily precipitate of
capreomycin separates. The supernatant is decanted and discarded, and the
oily precipitate which remains is dissolved in 20 L of distilled water. The
aqueous solution is filtered. The filtrate is added to 240 L of methanol. The
methanolic solution is acidified by the addition of 1 L of 10 N sulfuric acid,
whereupon the precipitation of the capreomycin disulfate commences. The
mixture is permitted to stand overnight. The supernant is removed by
decantation and filtering. The precipitate is washed with 10 L of methanol,
yield Capreomycin 2510 g.
brand name
Capastat Sulfate (Lilly).
Therapeutic Function
Antibacterial, Antitubercular
Pharmacology
Capreomycin is poorly absorbed from GI tract and so must be given parenterally. It is excreted mainly unchanged in the urine following glomerular filtration. Capreomycin is a used as a second-line agent in combination with other drugs. It appears to be particularly useful in multidrug regimens for the treatment of drugresistant tuberculosis, especially with streptomycin resistance. Capreomycin is associated with ototoxicity and nephrotoxicity, and these adverse effects can be severe in patients with preexisting renal insufficiency.
Clinical Use
Antibacterial agent in combination with other drugs:
Tuberculosis that is resistant to first-line drugs
Drug interactions
Potentially hazardous interactions with other drugs
Increased risk of nephrotoxicity and ototoxicity with
aminoglycosides and vancomycin.
Metabolism
Approximately 50% of a dose is excreted unchanged in the urine by glomerular filtration within 12 hours.
CAPREOMYCIN Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29810 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7724 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9639 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52849 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; | peter68@ptchemgroup.com | China | 35425 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49977 | 58 |
Related articles
- Mechanism of action of Capreomycin
- Capreomycin is an important reserve antibiotic for the treatment of tuberculosis and has been used for this purpose for more t....
- Mar 29,2022
View Lastest Price from CAPREOMYCIN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-28 | Capreomycin free base
11003-38-6
|
US $2500.00-1520.00 / mg | 10g | TargetMol Chemicals Inc. | |||
 |
2019-12-27 | CAPREOMYCIN
11003-38-6
|
US $1.00 / g | 1g | 99% | 10000KGS | Career Henan Chemical Co |
-

- Capreomycin free base
11003-38-6
- US $2500.00-1520.00 / mg
- TargetMol Chemicals Inc.
-

- CAPREOMYCIN
11003-38-6
- US $1.00 / g
- 99%
- Career Henan Chemical Co
11003-38-6(CAPREOMYCIN)Related Search:
1of4