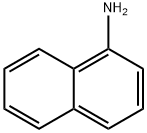
1-Naphthylamine
- CAS No.
- 134-32-7
- Chemical Name:
- 1-Naphthylamine
- Synonyms
- naphthalen-1-amine;1-AMINONAPHTHALENE;α-Naphthylamine;1-AMinonapthalene;1-Naphthalenamine;ALPHA-NAPHTHYLAMINE;1-Nap;ci37265;Aminogen I;C.I. 37265
- CBNumber:
- CB8719818
- Molecular Formula:
- C10H9N
- Molecular Weight:
- 143.19
- MDL Number:
- MFCD00004016
- MOL File:
- 134-32-7.mol
- MSDS File:
- SDS
| Melting point | 47-50 °C (lit.) |
|---|---|
| Boiling point | 301 °C (lit.) |
| Density | 1.114 g/mL at 25 °C (lit.) |
| vapor pressure | 0.003 hPa (20 °C) |
| refractive index | 1.6703 |
| Flash point | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | 1.7g/l |
| pka | 3.92(at 25℃) |
| form | flakes |
| color | light tan to purple |
| PH | 7.1 (1g/l, H2O, 20℃) |
| PH Range | Non& uorescence (3.4) to blue & uorescence (4.8) |
| Odor | Ammonia odor |
| Water Solubility | Insoluble. 0.1698 g/100 mL |
| Merck | 14,6398 |
| BRN | 386133 |
| Henry's Law Constant | 6.13 x 10-10 atm?m3/mol at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Exposure limits | TLV-TWA not assigned; carcinogenicity: Human Carcinogen (skin) (MSHA and OSHA). |
| Major Application | color filter, photoresists, recording media, light-emitting device, disk, display device, oil products, construction materials, leather, textile, chalk, explosives |
| CAS DataBase Reference | 134-32-7(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | 1-NAPHTHYLAMINE |
| FDA 21 CFR | 175.105 |
| EWG's Food Scores | 3-6 |
| FDA UNII | 9753I242R5 |
| Proposition 65 List | 1-Naphthylamine |
| NIST Chemistry Reference | 1-Naphthalenamine(134-32-7) |
| IARC | 3 (Vol. 4, Sup 7) 1987 |
| EPA Substance Registry System | 1-Naphthalenamine (134-32-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363-P405 | |||||||||
| Hazard Codes | C,N,Xn,T | |||||||||
| Risk Statements | 34-51/53-22-45 | |||||||||
| Safety Statements | 26-36/37/39-45-61-24-53 | |||||||||
| RIDADR | UN 2790 8/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | QM1400000 | |||||||||
| F | 8-23 | |||||||||
| Autoignition Temperature | 460 °C | |||||||||
| HS Code | 2921 45 00 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in Rabbit: 680 mg/kg | |||||||||
| NFPA 704 |
|
1-Naphthylamine price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22291 | 1-Naphthylamine for synthesis | 134-32-7 | 100g | $42.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22291 | 1-Naphthylamine for synthesis | 134-32-7 | 1kg | $157 | 2024-03-01 | Buy |
| Sigma-Aldrich | 38497 | Nitrate Reagent A for microbiology | 134-32-7 | 100mL | $45.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 34390 | 1-Naphthylamine analytical standard | 134-32-7 | 250mg | $116 | 2024-03-01 | Buy |
| TCI Chemical | N0052 | 1-Naphthylamine >99.0%(GC)(T) | 134-32-7 | 25g | $57 | 2024-03-01 | Buy |
1-Naphthylamine Chemical Properties,Uses,Production
Chemical Properties
α-Naphthylamine exists as white needle-like crystals which turn red on exposure to air. Has a weak ammonia-like odor. Solubility in water is 0.16% at 20℃. It behaves as a typical primary aromatic amine in forming salts with strong acids (but not with acetic or benzoic acid) and readily forming N-acyl derivatives. 1-Naphthylamine couples with diazo compounds in the 4-position, with up to 10 % byproduct being formed by coupling in the 2-position.
Physical properties
White to yellow crystals or rhombic needles with an unpleasant odor. Becomes purplish-red on exposure to air. Odor threshold concentrations were 140–290 μg/m3 (quoted, Keith and Walters, 1992).
Uses
1-Naphthylamine is used as a chemical intermediate in the synthesis of a wide variety of chemicals, the most important of which include dyes, tonic prints, Toning prints made with cerium salts, antioxidants, and herbicides.
Application
1-Naphthylamine is mainly used for the production of 1-naphthol. Other major uses are in the production of aminonaphthalenesulfonic acid dye intermediates and as a component of azo dyes.
1-Naphthylamine can also be used as a starting material to synthesize:
[(S)-HY-Phos], a novel chiral phosphine-phosphoramidite ligand for use in rhodium-catalyzed asymmetric hydrogenation of various functionalized olefins.
N-(naphthalen-1-yl)picolinamide.
Pitnot-2, an inactive analog of clathrin inhibitor Pitstop 2.
Preparation
1-Naphthylamine is prepared by reduction of 1-nitronaphthalene: Purified 1-nitronaphthalene was traditionally reduced with iron in boiling dilute hydrochloric acid, but modern plants use hydrogenation with a nickel catalyst. The 1-naphthylamine produced is further purified by distillation under vacuum. The content of 2-naphthylamine in the commercial product is specified at <10 ppm.
Definition
ChEBI: 1-naphthylamine is a naphthylamine that is naphthalene substituted by an amino group at position 1. It has a role as a human xenobiotic metabolite.
Synthesis Reference(s)
Tetrahedron Letters, 25, p. 429, 1984 DOI: 10.1016/S0040-4039(00)99903-9
Chemical and Pharmaceutical Bulletin, 34, p. 1524, 1986 DOI: 10.1248/cpb.34.1524
General Description
1-naphthylamine appears as a crystalline solid or a solid dissolved in a liquid. Insoluble in water and denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Sensitive to exposure to air and light. Insoluble in water. Napthyl amines can be slowly hydrolyzed, releasing NH3 as a byproduct [N.L. Drake, Org. React. 1, (1942), 105].
Reactivity Profile
1-Aminonaphthalene is incompatible with oxidizing agents. 1-Aminonaphthalene is also incompatible with nitrous acid. 1-Aminonaphthalene reduces warm ammoniacal silver nitrate. .
Hazard
Toxic, especially if containing the β isomer; a questionable carcinogen.
Health Hazard
1-Naphthylamine is a moderately toxic and cancer-causing substance. The toxic symptoms arising from oral intake or skin absorption of this compound include acute hemorrhagic cystitis, dyspnea, ataxia, dysuria, and hematuria. An intraperitoneal LD50 value in mice is 96 mg/kg. Inhalation of dusts or vapors is hazardous, showing similar symptoms. 1-Naphthylamine caused leukemia in rats. There is substantial evidence of its cancer-causing effects in animals and humans.
Fire Hazard
Special Hazards of Combustion Products: Toxic nitrogen oxides are produced in a fire.
Safety Profile
Confirmed carcinogen with experimental tumorigenic data. Along with p-naphthylamine and benzidine, it has been incriminated as a cause of urinary bladder cancer. Poison by subcutaneous and intraperitoneal routes. Moderately toxic by ingestion. Mutation data reported. Combustible when exposed to heat or flame. Incompatible with nitrous acid. To fight fire, use dry chemical, CO2, mist, spray. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
α-Naphthylamine is used as an intermediate in dye production; for manufacturing herbicides and antioxidants; in the manufacture of condensation colors, rubber, and in the synthesis of many chemicals, such as α-naphthol, sodium naphthionate; o-naphthionic acid; Neville and Winther’s acid; sulfonated naphthylamines, α-naphthylthiourea (a rodenticide); and N-phenyl- α-naphthylamine.
Carcinogenicity
1-Naphthylamine has been tested for carcinogenic activity in mice, hamsters, and dogs by oral administration, in newborn mice by SC injection, and in mice by bladder implantation. Most of these studies reported negative findings, while a few found marginal or equivocal results. In contrast, with the exception of bladder implantation study, 2-naphthylamine gave positive results in virtually all these studies. Mixed results were reported in various genotoxicity tests. 1-Naphthylamine was positive in the Ames test and in vitro chromosome aberration, negative in micronucleus, cell transformation, and recessive lethal mutation in Drosophila, and inconclusive in sister chromatid exchange assays.
Environmental Fate
Biological. 1-Naphthylamine added to three different soils was incubated in the dark at 23 °C
under a carbon dioxide-free atmosphere. After 308 d, 16.6 to 30.7% of the 1-naphthylamine added
to soil biodegraded to carbon dioxide (Graveel et al., 1986). Li and Lee (1999) investigated the
reaction of 10 mL of 7 mM 1-naphthylamine with 4 g of a Chalmers soil (pH: 6.5, 11.1% sand,
72.8% silt, 16.0% clay). After 120 h, the soil was washed with acetonitrile and the extractant
analyzed using GC/MS. The primary transformation product was a dimer tentatively identified as
N-(4-aminonaphthyl)-1-naphthylamine. The investigators hypothesized that the formation of this
compound and two other unidentified dimers was catalyzed by minerals present in the soil.
Heukelekian and Rand (1955) reported a 5-d BOD value of 0.89 g/g which is 34.6% of the
ThOD value of 2.57 g/g. In activated sludge inoculum, following a 20-d adaptation period, no
degradation was observed (Pitter, 1976).
Photolytic. Low et al. (1991) reported that the photooxidation of aqueous primary amine
solutions by UV light in the presence of titanium dioxide resulted in the formation of ammonium
and nitrate ions.
Chemical/Physical. Kanno et al. (1982) studied the aqueous reaction of 1-naphthylamine and
other substituted aromatic hydrocarbons (aniline, toluidine, 2-naphthylamine, phenol, cresol,
pyrocatechol, resorcinol, hydroquinone, and 1-naphthol) with hypochlorous acid in the presence of
ammonium ion. They reported that the aromatic ring was not chlorinated as expected but was
cleaved by chloramine forming cyanogen chloride. The amount of cyanogen chloride that formed
increased as the pH was lowered (Kanno et al., 1982).
At influent concentrations (pH 3.0) of 10, 1.0, 0.1, and 0.01 mg/L, the GAC adsorption capacities
were 250, 140, 79, and 44 mg/g, respectively. At pHs 7.0 and 9.0, the GAC adsorption capacities
were 360, 160, 75, and 34 mg/g at influent concentrations of 10, 1.0, 0.1, and 0.01 mg/L,
respectively (Dobbs and Cohen, 1980).
Shipping
UN2077 alpha-Naphthylamine, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. PGIII.
Purification Methods
Sublime the amine at 120o in a stream of nitrogen, then crystallise it from pet ether (b 60-80o), or absolute EtOH then diethyl ether. Dry it in vacuo in an Abderhalden pistol. It has also been purified by crystallisation of its hydrochloride (see below) from water, followed by liberation of the free base and distillation; it is finally purified by zone melting. The styphnate has m 181-182o (from EtOH). [Beilstein 12 III 2846, 12 IV 3009.] CARCINOGEN.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrous acid, organic anhydrides, isocyanates, aldehydes. Oxidizes on contact with air.
Waste Disposal
Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalyst, or thermal device. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
1-Naphthylamine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18779 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9214 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10310 | 58 |
Related articles
- Purity and Hazards of 1-Naphthylamine
- It was found that commercial 1-naphthylamine for industrial use contains about 4% 2-naphthylamine and practical grade reagents....
- Dec 21,2023
View Lastest Price from 1-Naphthylamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-25 | 1-Naphthylamine
134-32-7
|
US $30.00-10.00 / kg | 1kg | 0.99 | 20 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2024-10-11 | 1-Naphthylamine
134-32-7
|
US $10.60 / KG | 1KG | 99% | 5000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2023-07-27 | 1-Naphthylamine
134-32-7
|
US $10.00 / kg | 1kg | 99% | 1000tons | Henan Bao Enluo International TradeCo.,LTD |
-

- 1-Naphthylamine
134-32-7
- US $30.00-10.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- 1-Naphthylamine
134-32-7
- US $10.60 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- 1-Naphthylamine
134-32-7
- US $10.00 / kg
- 99%
- Henan Bao Enluo International TradeCo.,LTD