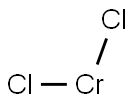
CHROMIUM(II) CHLORIDE
- CAS No.
- 10049-05-5
- Chemical Name:
- CHROMIUM(II) CHLORIDE
- Synonyms
- CRCL2;CHROMIUM DICHLORIDE;CHROME CHLORIDE;CHROMOUS CHLORIDE;chromous;Chromous acid;Chromium(II) chL;Chrome Dichloride;CHROMIUM(II) CHLORIDE;CHROMIUM(II) CHLORIDE
- CBNumber:
- CB8751238
- Molecular Formula:
- Cl2Cr
- Molecular Weight:
- 122.9
- MDL Number:
- MFCD00010947
- MOL File:
- 10049-05-5.mol
- MSDS File:
- SDS
| Melting point | 824 °C(lit.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 1120°C (estimate) | ||||||||||||||
| Density | 2.9 g/mL at 25 °C(lit.) | ||||||||||||||
| storage temp. | Store below +30°C. | ||||||||||||||
| solubility | soluble in H2O | ||||||||||||||
| form | powder | ||||||||||||||
| Specific Gravity | 2.878 | ||||||||||||||
| color | Off-white to green-gray | ||||||||||||||
| Water Solubility | Soluble in water. Insoluble in alcohol and ether. | ||||||||||||||
| Sensitive | Air & Moisture Sensitive | ||||||||||||||
| Crystal Structure | CaCl2 type | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Merck | 14,2244 | ||||||||||||||
| Space group | Pnnm | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | NIOSH: IDLH 250 mg/m3; TWA 0.5 mg/m3 | ||||||||||||||
| Stability | Stable, but decomposes in water or moist air. Incompatible with water, oxidizing agents, active metals. | ||||||||||||||
| CAS DataBase Reference | 10049-05-5(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 1 | ||||||||||||||
| FDA UNII | CET32HKA21 | ||||||||||||||
| EPA Substance Registry System | Chromium(II) chloride (10049-05-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P301+P312+P330 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GB5250000 | |||||||||
| F | 1-10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2827 39 85 | |||||||||
| Toxicity | LD50 orally in rats: 1.87 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
CHROMIUM(II) CHLORIDE price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.02482 | Chromium(II) chloride for synthesis | 10049-05-5 | 10g | $200 | 2024-03-01 | Buy |
| Sigma-Aldrich | 244805 | Chromium(II) chloride 95% | 10049-05-5 | 5g | $151 | 2024-03-01 | Buy |
| Sigma-Aldrich | 244805 | Chromium(II) chloride 95% | 10049-05-5 | 25g | $594 | 2024-03-01 | Buy |
| Alfa Aesar | 047176 | Chromium(II) chloride, 99.9% (metals basis) | 10049-05-5 | 1g | $36.65 | 2024-03-01 | Buy |
| Alfa Aesar | 047176 | Chromium(II) chloride, 99.9% (metals basis) | 10049-05-5 | 5g | $105 | 2024-03-01 | Buy |
CHROMIUM(II) CHLORIDE Chemical Properties,Uses,Production
Physical Properties
White lustrous needles or fibrous mass; hygroscopic; highly soluble in water, forming blue solution; insoluble in ether. The tetrahydrate occurs in blue hygroscopic crystalline form, that changes to green modification above 38°C; decomposes to trihydrate at 51°C; soluble in water.
Uses
Chromium(II) chloride is used as a reducing agent; as a catalyst in organic reactions; in chromium plating of metals; and as an analytical reagent for the dehalogenation of vic-dihalides. As a reducing agent, it is used to reduce alpha-haloketones to parent ketones, epoxides to olefins, chloroimides to imines, and aromatic aldehydes to corresponding alcohols.
Preparation
Chromium(II) chloride may be prepared by the reaction of chromium with anhydrous hydrogen chloride at 600 to 700°C:
Cr + 2HCl → CrCl2 + H2
Also, the compound may be prepared by the reduction of chromium(III) chloride with hydrogen at 500 to 600°C:
2CrCl3 + H2→ 2CrCl2 + 2HCl
An aqueous solution of chromium(II) chloride for organic reduction may be prepared as follows:
Amalgamate zinc by shaking 400 g zinc dust with a solution containing 32g HgCl2, 20 mL conc. HCl and 400 mL water. Decant the aqueous phase. To the amalgamated zinc add 800 mL water, 80 mL conc. HCl, and 200 g CrCl3•6H2O. Bubble CO2 through the solution to agitate it and prevent any possible reoxidation of chromium by air. The solution that turns light blue may be used in organic reduction.
Description
Chromous chloride is a white-to-blue solid orlustrous needles which turn blue in water. Molecularweight =122.90; Boiling point =1300℃; Meltingpoint =824℃. Hazard Identification (based on NFPA-704M Rating System): Health 1, Flammability 0, Reactivity 0.Soluble in water.
Chemical Properties
grey green powder
Chemical Properties
Chromous chloride is a white-to-bluish solid or lustrous needles which turn Blue in water
Uses
The vapor-phase co-reductions with other metal halides by hydrogen results in finely divided intermetallics with applications as structural materials or compounds with useful thermoelectric, magnetic, and oxidation-resistance properties.
Uses
Chromium(II) chloride is used as a precursor to other inorganic and organometallic chromium complexes. It is a reagent in the Nozaki-Hiyama-Kishi reaction. It is also used in the Takai olefination to prepare vinyl iodides from aldehydes in the presence of iodoform.
General Description
A white crystalline solid. The primary hazard is the threat to the environment. Immediate steps must be taken to prevent its spread to the environment. CHROMIUM(II) CHLORIDE is used to make other chemicals and as an oxygen absorbent.
Air & Water Reactions
Stable in dry air, but oxidizes rapidly in moist air or standing water with liberation of Hydrogen, [Merck, 11th ed., 1989]. Soluble in water.
Reactivity Profile
Inorganic reducing agents, such as CHROMIUM(II) CHLORIDE, react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Their reactions with oxidizing agents may be violent. Long storage of a CHROMIUM(II) CHLORIDE solution culminated in an explosion. This was ascribed to the slow buildup of hydrogen gas that was produced by reduction of water by the Cr(II) ion [MCA Case History No. 1660].
Health Hazard
DUST: Harmful if inhaled. SOLID: Irritating to skin and eyes. Harmful if swallowed.
Potential Exposure
It is used in metal alloys and metal finishing; textile treatment including mothproofing, waterproofing, printing; and dying, leather tanning; making photographic chemicals; and green pigments for various uses.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Note to physician: In case of fume inhalation, treat pulmonary edema. Consider administering prednisone or othercorticosteroid to reduce tissue response to fume. Positivepressure ventilation may be necessary. Treat metal fumefever with bed rest, analgesics, and antipyretics.
storage
Color Code—Yellow Stripe (strong reducingagent): Reactivity Hazard; Store separately in an area isolated from flammables, combustibles, or other yellow-codedmaterials. Prior to working with chromous chloride youshould be trained on its proper handling and storage. Storein tightly closed containers in a cool, well-ventilated areaaway from water and moisture. Where possible, automatically pump liquid from drums or other storage containers toprocess containers.
Shipping
UN3260 Corrosive solid, acidic, inorganic, n.o. s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Purification Methods
It is obtained from the dihydrate by heating in vacuo at 180o. It is a very hygroscopic white powder which dissolves in H2O to give a sky blue solution. It is stable in dry air but oxidises rapidly in moist air and should be stored in air tight containers. It sublimes at 800o in a current of HCl gas and should be cooled in the presence of HCl gas. Alternatively it can be washed with air-free Et2O and dried at 110-120o. [Burg Inorg Synth III 150 1950, Balthis & Bailar (4 H2O) Inorg Synth I 125 1939, Hein & Herzog in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II pp 1336-1338 1965.]
Incompatibilities
Very hygroscopic. The substance is a strong reducing agent. Reacts with oxidizers. Reacts with water, forming flammable hydrogen gas
CHROMIUM(II) CHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29881 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 13417 | 58 |
View Lastest Price from CHROMIUM(II) CHLORIDE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-07 | Chromium Chloride
10049-05-5
|
US $0.00 / kg | 1kg | 99% | 1000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2021-07-02 | CHROMIUM(II) CHLORIDE
10049-05-5
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2019-07-06 | CHROMIUM(II) CHLORIDE
10049-05-5
|
US $1.00 / KG | 1G | 98% | 100KG | Career Henan Chemical Co |
-

- Chromium Chloride
10049-05-5
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- CHROMIUM(II) CHLORIDE
10049-05-5
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-
- CHROMIUM(II) CHLORIDE
10049-05-5
- US $1.00 / KG
- 98%
- Career Henan Chemical Co
10049-05-5(CHROMIUM(II) CHLORIDE)Related Search:
1of4