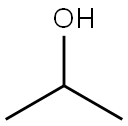
Isopropyl alcohol
- CAS No.
- 67-63-0
- Chemical Name:
- Isopropyl alcohol
- Synonyms
- IPA;2-Propanol;Isopropanol;i-PrOH;iPrOH;i-Propanol;Propanol-2;2-PROPANOL (IPA);67-73-0;lsopropanol
- CBNumber:
- CB8854102
- Molecular Formula:
- C3H8O
Lewis structure
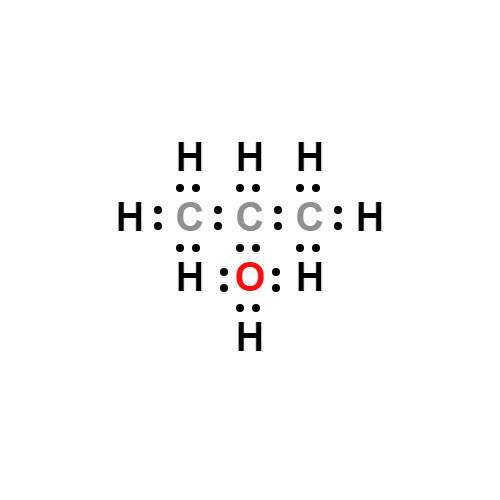
- Molecular Weight:
- 60.1
- MDL Number:
- MFCD00005913
- MOL File:
- 67-63-0.mol
- MSDS File:
- SDS
| Melting point | -89.5 °C |
|---|---|
| Boiling point | 82 °C(lit.) |
| Density | 0.785 g/mL at 25 °C(lit.) |
| vapor density | 2.1 (vs air) |
| vapor pressure | 33 mm Hg ( 20 °C) |
| FEMA | 2929 | ISOPROPYL ALCOHOL |
| refractive index |
n |
| Flash point | 53 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | water: soluble (completely) |
| pka | 17.1(at 25℃) |
| form | Low Melting Solid |
| Specific Gravity | approximate 0.785(20/20℃)(Ph.Eur.) |
| color | colorless |
| Relative polarity | 0.546 |
| Odor | Like ethyl alcohol; sharp, somewhat unpleasant; characteristic mild alcoholic; nonresidual. |
| explosive limit | 2-13.4%(V) |
| Odor Threshold | 26ppm |
| Odor Type | alcoholic |
| Evaporation Rate | 2.83 |
| Water Solubility | miscible |
| FreezingPoint | -89.5℃ |
| λmax |
λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.01 |
| Merck | 14,5208 |
| JECFA Number | 277 |
| BRN | 635639 |
| Exposure limits | TLV-TWA 980 mg/m3 (400 ppm); STEL 1225 mg/m3 (500 ppm) (ACGIH); IDLH 12,000 ppm (NIOSH). |
| Dielectric constant | 18.0(Ambient) |
| Stability | Volatile |
| LogP | 0.050 |
| CAS DataBase Reference | 67-63-0(CAS DataBase Reference) |
| FDA 21 CFR | 173.240; 172.515; 173.340; 175.105; 176.200; 177.1200; 177.2800; 178.3910; 310.545; 73.1; 73.1001; 73.345; 73.615 |
| Substances Added to Food (formerly EAFUS) | ISOPROPYL ALCOHOL |
| EWG's Food Scores | 2 |
| FDA UNII | ND2M416302 |
| NCI Drug Dictionary | isopropyl alcohol |
| ATC code | D08AX05 |
| IARC | 3 (Vol. 15, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Isopropyl alcohol(67-63-0) |
| EPA Substance Registry System | Isopropanol (67-63-0) |
| Pesticides Freedom of Information Act (FOIA) | Isopropanol |
| Cosmetics Info | Isopropyl Alcohol |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319-H336 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,F,Xn | |||||||||
| Risk Statements | 11-36-67-40-10-36/38 | |||||||||
| Safety Statements | 7-16-24/25-26-36/37 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 400 ppm (980 mg/m3), STEL: 500 ppm (1225 mg/m3) | |||||||||
| RIDADR | UN 1219 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | NT8050000 | |||||||||
| F | 3-10 | |||||||||
| Autoignition Temperature | 750 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2905 12 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| Hazardous Substances Data | 67-63-0(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 5.8 g/kg (Smyth, Carpenter) | |||||||||
| IDLA | 2,000 ppm [10% LEL] | |||||||||
| NFPA 704 |
|
Isopropyl alcohol price More Price(228)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W292912 | Isopropyl alcohol natural, ≥98%, FG | 67-63-0 | 1kg | $125 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292912 | Isopropyl alcohol natural, ≥98%, FG | 67-63-0 | 5kg | $447 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292907 | Isopropyl alcohol ≥99.7%, FCC, FG | 67-63-0 | 1kg | $76.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292907 | Isopropyl alcohol ≥99.7%, FCC, FG | 67-63-0 | 8kg | $180 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292907 | Isopropyl alcohol ≥99.7%, FCC, FG | 67-63-0 | 20kg | $311 | 2024-03-01 | Buy |
Isopropyl alcohol Chemical Properties,Uses,Production
description
Isopropanol is also known as isopropyl alcohol. It is the simplest secondary alcohol and is one of the isomers of n-propanol. It is a kind of flammable liquid which is colorless with strong smell being similar to the smell of the mixture of ethanol and acetone. It is soluble in water, alcohol, ether, benzene, chloroform and most organic solvents and is miscible with water, alcohol, ether and can form azeotrope with water. Density (specific gravity): 0.7863g/cm3, melting point:-88.5 ℃, boiling point: 82.5 ℃, flash point: 11.7 ℃, ignition point: 460 ℃, refractive index: 1.3772. Its vapor can cause slight irritation on the eyes, nose and throat; it can be absorbed through the skin. Its vapor can form explosive mixture with air. Its explosion limit is 2.0% to 12% (by volume). It belongs to a moderate explosive hazardous material and flammable, low toxic substance. The toxicity of its vapors is twice as high as ethanol while oral administration causes the opposite toxicity.
Figure 1 is the structural formula of isopropanol.
In many cases, isopropanol can substitute ethanol as the solvent and is a good solvent and chemical raw materials which can be applied to not only painting, pharmaceuticals, pesticides, cosmetics and other industries, but also the production of acetone, isopropyl ester, isopropylamine (the raw material for production of atrazine), di-isopropyl ether, isopropyl acetate and thymol crystal etc. It was the first product which is made from the petroleum raw material in the history of the development of petrochemicals.
Production Process
In 1855, Frenchman M. Berthelot first reported the production of isopropanol through the hydration reaction between propylene and sulfuric acid, called indirect hydration. In 1919, the Americans C. Ellis had conducted industrial development on this. At the end of 1920, the American Standard Oil Company of New Jersey adopted the approach of Ellis Act and established the production equipment for putting into formal production. In 1951, the British company Imperial Chemical Industries began to produce isopropanol with the direct hydration method from propylene. Since then, many countries have used this method and made related improvements.
Indirect hydration reaction: propylene is first reacted with sulfuric acid to obtain isopropyl hydrogen sulfate, which generates isopropanol after hydrolysis, and the reaction of the formula:
CH3CH = CH2 + H2SO4 → (CH3) 2CHOSO3H
(CH3) 2CHOSO3H + H2O─ → (CH3) 2CHOH + H2SO4
the concentration of the applied sulfuric acid is generally greater than 60% (by mass), and the reaction is conducted at 2~2.8MPa and 60~65 ° C; The hydrolysis reaction happens at slight increased pressure and at below 30 ° C.
Direct hydration: propylene directly has hydration reaction with water in the presence of a catalyst upon heating and increased pressure to generate isopropanol with a selectivity of 96%. Reaction is: CH3CH = CH2 + H2O → (CH3) 2CHOH; the used catalyst includes tungsten compound, phosphate and ion exchange resin; the commonly used catalyst is phosphoric acid catalyst with carrier (see solid acid catalyst) with conditions of 2~6MPa, 240~260 ° C. Compared with the indirect method, this method does not have issue regarding to sulfuric acid corrosion and dilute acid concentration and therefore, it dominant in industrial production.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Uses
Isopropyl alcohol is an important chemical products and raw materials. It is mainly applied to various fields including pharmaceutical, cosmetics, plastics, fragrances, paint as well as being used as the dehydrating agent and cleaning agent in and electronics industry. It can also be used as the reagent for determination of barium, calcium, magnesium, nickel, potassium, sodium and strontium. It can also be used as the reference material of chromatographic analysis.
In the manufacturing industry of circuit board, it is used as a cleaning agent, and the production of PCB holes for conductivity. Many people find that it can not only clean the motherboard with excellent performance, but also get the best results. In addition, it is used for other electronic devices, including cleaning disc cartridge, floppy disk drives, magnetic tape, and the laser tip of the disc driver of CD or DVD player.
Isopropyl alcohol can also be used as the solvent of oil and gel as well as for the manufacture of fishmeal feed concentrate. Low-quality isopropanol can also be used in automotive fuels. As the raw material of production of acetone, the usage amount of isopropanol is reducing. There are several compounds which are synthesized from isopropanol, such as isopropyl ester, methyl isobutyl ketone, di-isopropylamine, di-isopropyl ether, isopropyl acetate, thymol and many kinds of esters. We can supply isopropanol of different quality depending on the end use it. The conventional quality of anhydrous isopropanol is more than 99%, while the special grade isopropanol content is higher than 99.8% (for flavors and drugs).
Toxicity
ADI value is not specified (FAO/WHO, 2001).
LD5050: 45rag/kg (rat, oral).
Limited use
FEMA (mg/kg): soft drinks: 25; sweets: 10 to 75; baked good: 75.
Description
Isopropanol is a clear, colorless alcohol that is used in the production of acetone and as a solvent in the manufacture of various industrial and commercial products. It is used by the public for a number of different purposes and is commonly known as rubbing alcohol. It is flammable and miscible with both water and many different organic solvents. Isopropanol can be prepared via three different methods: indirect hydration of propylene (the ‘strong acid’ method), direct hydration of propylene, and catalytic hydrogenation of acetone.
Chemical Properties
Isopropyl alcohol is a clear, colorless, mobile, volatile, flammable liquid with a characteristic, spirituous odor resembling that of a mixture of ethanol and acetone; it has a slightly bitter taste.It is miscible with water, ethyl ether, and ethyl alcohol. Isopropyl alcohol is incompatible with strong oxidizers, acetaldehyde, chlorine, ethylene oxide, acids, and isocyanates.
Occurrence
Reported found in apple and cognac aromas (esterified). Also found in apple, banana, grapefruit and lime juice, melon, papaya, pear, onion, peas, rutabaga, tomato, wheat bread, cheeses, milk, boiled egg, cooked beef, pork and mutton, hop oil beer, rum, cocoa, coffee, scotch whiskey, grape wines, peanut, pecan, soybean, honey, beans, plum brandy, walnut, crab, clam, prickly pear and clary sage.
Uses
Isopropyl alcohol is used in the production of acetone, isopropyl halides, glycerin, and aluminum isopropoxide; employed widely as an industrial solvent for paints, polishes, and insecticides; as an antiseptic (rubbing alcohol); and in organic synthesis for introducing the isopropyl or isopropoxy group into the molecule. Being a common laboratory solvent like methanol, the exposure risks are always high; however, its toxicity is comparatively low.
Preparation
Isopropyl alcohol may be prepared from propylene; by the catalytic reduction of acetone, or by fermentation of certain carbohydrates.
Definition
ChEBI: Isopropyl Alcohol is a secondary alcohol that is propane in which one of the hydrogens attached to the central carbon is substituted by a hydroxy group. It is an isomer of propyl alcohol with antibacterial properties.
Application
Isopropyl Alcohol is used in a variety of applications including as a solvent for industrial processes and coating; as a component in cleaning, car care and deicing products; as a wetting agent for printing inks and as a feedstock in the manufacture of ester and Mogas/Luboil additives.
isopropyl alcohol is a carrier, anti-bacterial, and solvent for skin care lotions. Isopropyl alcohol is made from propylene, a petroleum derivative.
When compared to ethanol, 50% less is required for nucleic acid precipitation, thus minimizing the total volume to be centrifuged for DNA or RNA recovery.
Isopropyl alcohol 70% is used as an ingredient in alcohol swabs and alcohol wipes for wound cleaning, it is found in hand sanitizers, and in ear drops to prevent swimmer's ear.
Aroma threshold values
Detection: 40 to 601 ppm
General Description
Volatile, colorless liquid with a sharp musty odor like rubbing alcohol. Flash point of 53°F. Vapors are heavier than air and mildly irritating to the eyes, nose, and throat. Density approximately 6.5 lb / gal. Used in making cosmetics, skin and hair preparations, pharmaceuticals, perfumes, lacquer formulations, dye solutions, antifreezes, soaps, window cleaners. Sold in 70% aqueous solution as rubbing alcohol.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
Isopropyl Alcohol can react with AIR and OXYGEN over time to form unstable peroxides that can explode. Contact with 2-butanone increases the rate of peroxide formation. An explosive reaction occurs when Isopropanol is heated with (aluminum isopropoxide + crotonaldehyde). Forms explosive mixtures with trinitromethane and hydrogen peroxide. Reacts with barium perchlorate to form a highly explosive compound. Ignites on contact with dioxygenyl tetrafluoroborate, chromium trioxide and potassium-tert-butoxide. Vigorous reactions occur with (hydrogen + palladium), nitroform, oleum, COCl2, aluminum triisopropoxide and oxidizing agents. Reacts explosively with phosgene in the presence of iron salts. Incompatible with acids, acid anhydrides, halogens and aluminum . Isopropanol can react with PCl3, forming toxic HCl gas. (Logsdon, John E., Richard A. Loke., sopropyl Alcohol. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 1996.).
Health Hazard
Exposures to isopropyl alcohol cause irritation to the eyes and mucous membranes. Exposures to isopropyl alcohol for 3–5 min (400 ppm) caused mild irritation of the eyes, nose, and throat, and at 800 ppm these symptoms became severe. Ingestion or an oral dose of 25 mL in 100 mL of water produced hypotension, facial flushing, bradycardia, and dizziness. Ingestion in large quantities caused extensive hemorrhagic tracheobronchitis, bronchopneumonia, and hemorrhagic pulmonary edema. Prolonged skin contact with isopropyl alcohol caused eczema and sensitivity. Delayed dermal absorption is attributed to a number of pediatric poisonings that have occurred following repeated or prolonged sponge bathing with isopropyl alcohol to reduce fever. In several cases, symptoms included respiratory distress, stupor, and coma. Laboratory animals exposed to isopropyl alcohol develop poisoning with symptoms of hind leg paralysis, unsteadiness, lack of muscular coordination, respiratory depression, and stupor. Isopropyl alcohol is a potent CNS depressant, and in large doses causes cardiovascular depression.
Fire Hazard
Isopropyl Alcohol(IPA) is highly flammable in its liquid and vapor forms and flammable atmospheres can be created at temperatures as low as 540°F /120℃ . This means that any environment where IPA is being used needs to be well ventilated. It should be kept away from heat and open flame. As the vapour is heavier than air, it may spread along the ground, so care needs to be taken that the vapour is not ignited by a distant source.
Pharmaceutical Applications
Isopropyl alcohol (propan-2-ol) is used in cosmetics and pharmaceutical
formulations, primarily as a solvent in topical formulations.( It is not recommended for oral use owing to its toxicity.
Although it is used in lotions, the marked degreasing properties
of isopropyl alcohol may limit its usefulness in preparations used
repeatedly. Isopropyl alcohol is also used as a solvent both for tablet
film-coating and for tablet granulation, where the isopropyl
alcohol is subsequently removed by evaporation. It has also been
shown to significantly increase the skin permeability of nimesulide
from carbomer 934.
Isopropyl alcohol has some antimicrobial activity and a 70% v/v aqueous solution is used as a topical disinfectant.
Therapeutically, isopropyl alcohol has been investigated for the
treatment of postoperative nausea or vomiting.
Safety
Isopropyl alcohol is about twice as toxic as ethanol and should therefore not be administered orally; isopropyl alcohol also has an unpleasant taste. Symptoms of isopropyl alcohol toxicity are similar to those for ethanol except that isopropyl alcohol has no initial euphoric action, and gastritis and vomiting are more prominent; see Alcohol. Delta osmolality may be useful as rapid screen test to identify patients at risk of complications from ingestion of isopropyl alcohol. The lethal oral dose is estimated to be about 120–250mL although toxic symptoms may be produced by 20 mL.
Adverse effects following parenteral administration of up to 20mL of isopropyl alcohol diluted with water have included only a sensation of heat and a slight lowering of blood pressure. However, isopropyl alcohol is not commonly used in parenteral products.
Although inhalation can cause irritation and coma, the inhalation of isopropyl alcohol has been investigated in therapeutic applications.
Isopropyl alcohol is most frequently used in topical pharmaceutical formulations where it may act as a local irritant. When applied to the eye it can cause corneal burns and eye damage.
LD50 (dog, oral): 4.80 g/kg
LD50 (mouse, oral): 3.6 g/kg
LD50 (mouse, IP): 4.48 g/kg
LD50 (mouse, IV): 1.51 g/kg
LD50 (rabbit, oral): 6.41 g/kg
LD50 (rabbit, skin): 12.8 g/kg
LD50 (rat, IP): 2.74 g/kg
LD50 (rat, IV): 1.09 g/kg
LD50 (rat, oral): 5.05 g/kg
Synthesis
Synthetically prepared from acetylene or propylene.
Carcinogenicity
CD-1 mice were exposed by inhalation to 0, 500, 2500, or 5000 ppm of isopropanol vapor for 6 h/day, 5 days/week for 18 months. An additional group of mice (all exposure levels) were assigned to a recovery group that were exposed to isopropanol for 12 months and then retained until study termination at 18 months. There was no increased frequency of neoplastic lesions in any of the isopropanol-exposed animals. Nonneoplastic lesions were limited to the testes (males) and the kidney. In the testes, enlargement of the seminal vesicles occurred in the absence of associated inflammatory or degenerative changes. The kidney effects included tubular proteinosis and/or tubular dilatation. The incidence of testicular and kidney effects was not increased in the isopropanol-exposed recovery animals.
Environmental Fate
The vast majority of isopropanol in the environment originates from manufacturing processes. Small amounts are produced by certain microbes, fungi, and yeast. The high volatility of isopropanol ensures that when it is released into the environment in any state, it eventually ends up in the atmosphere. There, it can be degraded by hydroxyl radicals or it can return to soil or water through precipitation. Its half-life in the environment is approximately 3.2 days and is highly biodegradable; bioaccumulation in plants and animals does not occur.
storage
Isopropyl alcohol should be stored in a cool, dry, well-ventilated area in tightly sealed containers with a proper label. Outside or detached storage is preferable. Inside storage should be a flammable liquids storage room or cabinet. Workers should not store isopropyl alcohol above 37°C (100°F). Containers of isopropyl alcohol should be protected from physical damage and contact with air, and should be stored separately from strong oxidizers, acetaldehyde, chlorine, ethylene oxide, acids, and isocyanates. Isopropyl alcohol should be transported to the nearest laboratory as quickly as possible in cool containers.
Purification Methods
Isopropyl alcohol is prepared commercially by dissolution of propene in H2SO4, followed by hydrolysis of the sulfate ester. Major impurities are water, lower alcohols and oxidation products such as aldehydes and ketones. Purification of isopropanol follows substantially the same procedure as for n-propyl alcohol. Isopropanol forms a constant-boiling mixture, b 80.3o, with water. Most of the water can be removed from this 91% isopropanol by refluxing with CaO (200g/L) for several hours, then distilling. The distillate can be dried further with CaH2, magnesium ribbon, BaO, CaSO4, calcium, anhydrous CuSO4 or Linde type 5A molecular sieves. Distillation from sulfanilic acid removes ammonia and other basic impurities. Peroxides [indicated by liberation of iodine from weakly acid (HCl) solutions of 2% KI] can be removed by refluxing with solid stannous chloride or with NaBH4 then the alcohol is fractionally distilled. To obtain isopropanol containing only 0.002M of water, sodium (8g/L) is dissolved in material dried by distillation from CaSO4. Isopropyl benzoate (35mL) is then added and, after refluxing for 3hours, the alcohol is distilled through a 50-cm Vigreux column (p 11). [Hine & Tanabe J Am Chem Soc 80 3002 1958.] Other purification steps for isopropanol include refluxing with solid aluminium isopropoxide, refluxing with NaBH4 for 24hours, and removing acetone by treatment with, and distillation from, 2,4-dinitrophenylhydrazine. Peroxides re-form in isopropanol if it is kept for several days in contact with air. [Beilstein 1 IV 1461.]
Toxicity evaluation
Isopropanol is similar to other alcohols in its ability to induce central nervous system (CNS) depression by enhancing inhibitory neuronal activity and antagonizing excitatory neuronal activity. It also can cause localized irritation upon contact with skin and mucous membranes after dermal exposure and ingestion, respectively.
Incompatibilities
Incompatible with oxidizing agents such as hydrogen peroxide and nitric acid, which cause decomposition. Isopropyl alcohol may be salted out from aqueous mixtures by the addition of sodium chloride, sodium sulfate, and other salts, or by the addition of sodium hydroxide.
Precautions
Workers should wash hands and face thoroughly after handling isopropyl alcohol. Workers should wear gloves, safety glasses and a face shield, boots, apron, and a full impermeable suit is recommended if exposure is possible to a large portion of the body.
Regulatory Status
Included in the FDA Inactive Ingredients Database (oral capsules, tablets, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Isopropyl alcohol Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| Deoksan General Science Co., Ltd | +82-02-957-3792 +82-02-957-3792 | dscsi@hanmail.net | South Korea | 4 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; | peter68@ptchemgroup.com | China | 35425 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 990 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| Henan Xiangduo Industry Co., Ltd. | +86-15981848961 +86-15981848961 | sales@xiangduochem.com | China | 497 | 58 |
| Runte International Trade Limited | 19565631292 19565631292 | lucky@sdruntechem.com | China | 270 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 | admin@china-yime.com | China | 563 | 58 |
Related articles
- Difference Between Isopropyl Alcohol and Acetone?
- The key difference between acetone and isopropyl alcohol is that acetone has a C=O bond in the middle of the chemical structur....
- Mar 8,2024
- Molecular polarity and non-polarity of isopropyl alcohol
- Isopropyl alcohol, also known as 2-propanol, is a versatile organic compound with various chemical properties. It has a molecu....
- Dec 28,2023
- Isopropyl Alcohol: Applications, Toxicity and Production
- Isopropyl alcohol is a clear, colorless, flammable liquid with a distinct odor.
- May 9,2023
View Lastest Price from Isopropyl alcohol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-13 | Isopropyl alcohol
67-63-0
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-12-12 | Isopropyl alcohol
67-63-0
|
US $1140.00-1130.00 / tons | 10tons | 99% | 1000tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-11-07 | Isopropyl alcohol
67-63-0
|
US $8.00 / kg | 1kg | 99% | 20000 | Hebei Jingbo New Material Technology Co., Ltd |
-

- Isopropyl alcohol
67-63-0
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Isopropyl alcohol
67-63-0
- US $1140.00-1130.00 / tons
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Isopropyl alcohol
67-63-0
- US $8.00 / kg
- 99%
- Hebei Jingbo New Material Technology Co., Ltd
67-63-0(Isopropyl alcohol)Related Search:
1of4