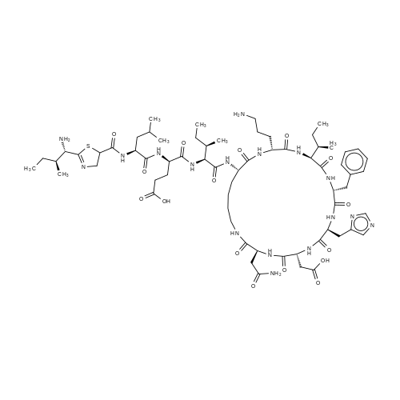
Bacitracin
- CAS No.
- 1405-87-4
- Chemical Name:
- Bacitracin
- Synonyms
- BACITRACIN A;septa;Ziba-RX;baciguent;ayfivin;Agfivin;baci-jel;usafcb-7;BACIDRIN;ALTRACIN
- CBNumber:
- CB9165863
- Molecular Formula:
- C66H102N17O16S
- Molecular Weight:
- 1421.68538
- MDL Number:
- MFCD27976769
- MOL File:
- 1405-87-4.mol
- MSDS File:
- SDS
| Melting point | 221-225°C |
|---|---|
| Density | 1.0166 (rough estimate) |
| refractive index | 1.6700 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear to very slightly hazy, deep yellow |
| form | Powder |
| color | white to faintly beige |
| Odor | odorless or acetic odor |
| Water Solubility | soluble |
| Merck | 13,936 |
| LogP | -0.050 (est) |
| FDA 21 CFR | 556.70 |
| EWG's Food Scores | 1 |
| FDA UNII | 58H6RWO52I |
| NCI Drug Dictionary | bacitracin |
| ATC code | D06AX05,J01XX10,R02AB04 |
| EPA Substance Registry System | Bacitracin (1405-87-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317 |
| Precautionary statements | P260-P271-P272-P280g-P302+P352-P304+P340-P312-P363-P403+P233-P501c |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22 |
| Safety Statements | 22-24/25-45-36 |
| WGK Germany | 2 |
| RTECS | CP0175000 |
| F | 21 |
| TSCA | Yes |
| HS Code | 29419090 |
| Toxicity | LD50 oral in guinea pig: 2gm/kg |
Bacitracin price More Price(33)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B0125 | Bacitracin from Bacillus licheniformis, ≥65?IU/mg | 1405-87-4 | 50000units | $53.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | B0125 | Bacitracin from Bacillus licheniformis, ≥65?IU/mg | 1405-87-4 | 250000units | $94.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | B0125 | Bacitracin from Bacillus licheniformis, ≥65?IU/mg | 1405-87-4 | 1250000units | $367 | 2024-03-01 | Buy |
| Sigma-Aldrich | 11702 | Bacitracin from | 1405-87-4 | 5g | $98.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 11702 | Bacitracin from | 1405-87-4 | 25g | $363 | 2024-03-01 | Buy |
Bacitracin Chemical Properties,Uses,Production
Mode of action
The lipid carrier involved in transporting the cell wall building block across the membrane is a C55 isoprenyl phosphate. The lipid acquires an additional phosphate group in the transport process and must be dephosphorylated in order to regenerate the native compound for another round of transfer. Bacitracin as cyclic peptide antibiotics binds to the C55 lipid carrier. Bacitracin inhibits its dephosphorylation thus disrupting the lipid carrier cycle.
Description
Bacitracin is a mixture of similar peptides produced by fermentation of the bacterium Bacillus subtil is. The A-type component predominates. Its mode of action is to inhibit both peptidoglycan biosynthesis at a late stage (probably at the dephosphorylation of the phospholipid carrier step) and disruptions of plasma membrane function.
Chemical Properties
White to pale buff powder
Chemical Properties
Bacitracin is a white to light tan powder which is odorless or having a slight odor and very bitter taste.
Originator
Baciguent,Upjohn,US,1948
Uses
antibiotic agent effective against gram-positive organisms and spirochetes; in products for topieal treatment, ear medications and ophthalmie drugs
Uses
Polypeptide antibacterial.
Uses
Bacitracin is a peptide antibiotic effective against gram-postive bacteria. Bacitracin is an inhibitor of peptidoglycan synthesis. Bacitracin disrupts bacterial cell wall synthesis by inhibiting depho sphorylation of lipid pyrophosphate. Bacitracin also strongly inhibits proline endopeptidase from human muscle.
Definition
A complex of cyclic peptide antibiotic of known chemical structure isolated from the Tracy-I strain of Bacillus subtilis.
Indications
This polypeptide antibiotic, which is produced from Bacillus subtilis,
interferes with bacterial cell wall growth and is bactericidal against many grampositive
organisms such as streptococci, staphylococci, and pneumococci but is
inactive against most gram-negative organisms. All anaerobic cocci, Neisseria, and
the tetanus and diphtheria bacilli are also sensitive to bacitracin.
Resistance is rare,
but some staphylococcal strains are inherently resistant. Hypersensitivity reactions
are uncommon. Bacitracin is stable in petrolatum (but not water-miscible preparations)
and is available as an ointment or as a component of antibiotic mixtures.
Sensitization to bacitracin has been reported more recently, particularly in patients
with leg ulcers.
Manufacturing Process
The early patent, US Patent 2,498,165 first disclosed bacitracin and described
a process for preparing bacitracin, comprising cultivating Bacillus subtilis Tracy
I in a nutritive medium, at substantially pH 7 and 37°C, for more than three
days, extracting the antibiotic from the resulting medium with a low molecular
weight alcohol, concentrating the resulting alcoholic solution in vacuo,
acidifying the resulting concentrate, extracting the antibiotic from the resulting
solution, and precipitating the antibiotic from the resulting solution, with a
precipitating agent for the antibiotic, selected from the group consisting of
Reinecke's salt, phosphotungstic acid, phosphomolybdic acid, molybdic acid,
picric acid, ammonium rhodanilate, and azobenzene-p-sulfonicacid.
A subsequent patent, US Patent 2,828,246 described a commercial process for
bacitracin production. A 1,230 gallon portion of a medium containing 10%
soybean oil meal, 2.50% starch and 0.50% calcium carbonate having a pH of
7.0 was inoculated with a culture of bacitracin-producing bacteria of the
Bacillus subtilis group and the inoculated medium incubated for a period of 24
hours with aeration such that the superficial air velocity was 12.1. An assay of
the nutrient medium following the fermentation revealed a yield of bacitracin
amounting to 323 units/ml. This was more than twice the yields previously
obtained.
Then, a patent, US Patent 2,834,711 described the purification of bacitracin.
In this process for purifying bacitracin, the steps comprise adding a watersoluble
zinc salt to a partially purified aqueous solution of bacitracin, adjusting
the pH to from 5 to 9, recovering the precipitate which forms, dissolving the
precipitate in water at a pH not substantially in excess of 4, and removing the
zinc ion by passing the aqueous solution through a cation exchange resin and
drying the resulting solution to obtain dry solid bacitracin.
Another patent, US Patent 2,915,432describes a process of recovering and
concentrating bacitracin from aqueous filtered fermentation broth containing
on the order of 3% protein-aceous solids which comprises intimately
contacting the broth with a synthetic organic cation exchange resin having as
its functional groups nuclear sulfonic acids and having a crosslinkage of the
order of 1 to 2%, with the resin being in the hydrogen form, and eluting the
adsorbed bacitracin from the resin with a weak base.
Bacitracin recovery is described in US Patents 3,795,663 and 4,101,539.
brand name
Ziba (X Gen).
Therapeutic Function
Antibacterial
General Description
The organism from which Johnson et al. produced bacitracinin 1945 is a strain of B. subtilis. The organism hadbeen isolated from debrided tissue from a compound fracturein 7-year-old Margaret Tracy, hence the name “bacitracin.”Bacitracin is now produced from the licheniformisgroup (B. subtilis). Like tyrothricin, the first useful antibioticobtained from bacterial cultures, bacitracin is a complex mixture of polypeptides. So far, at least 10 polypeptideshave been isolated by countercurrent distribution techniques:A, A1, B, C, D, E, F1, F2, F3, and G. The commercialproduct known as bacitracin is a mixture of principallyA, with smaller amounts of B, D, E, and F1–3.The official product is a white to pale buff powder that isodorless or nearly so.
The activity of bacitracin is measured in units per milligram.The potency per milligram is not less than40 units/mg except for material prepared for parenteral use,which has a potency of not less than 50 units/mg. It is a bactericidalantibiotic that is active against a wide variety ofGram-positive organisms, very few Gram-negative organisms,and some others. It is believed to exert its bactericidaleffect through inhibition of mucopeptide cell wall synthesis.Its action is enhanced by zinc. Although bacitracin hasfound its widest use in topical preparations for local infections,it is quite effective in several systemic and local infectionswhen administered parenterally. It is not absorbedfrom the GI tract; accordingly, oral administration is withouteffect, except for the treatment of amebic infectionswithin the alimentary canal.
Hazard
Poison; moderately toxic; mutagen.
Pharmaceutical Applications
A mixture of peptides produced by Bacillus licheniformis.
Bacitracin A is the major constituent of commercial preparations.
The more stable zinc salt is used in topical formulations.
It has been widely used as a growth promoter in animals, but
has been banned for that purpose in the European Union.
It is highly active against many Gram-positive bacteria
and is mainly used as a component of topical preparations.
Although strains of Staph. aureus are usually susceptible,
they are rather less so than most other Gram-positive bacteria.
Streptococcus pyogenes is so much more susceptible than
other hemolytic streptococci that bacitracin susceptibility is
used as a screening test for identification. Clostridium difficile
and Actinomyces spp. are susceptible, but enterobacteria and
Pseudomonas spp. are resistant. Entamoeba histolytica is inhibited
by 0.6–10 mg/L.
Resistance is uncommon, but has been detected in Staph.
aureus following topical treatment.
It is nephrotoxic and unsuitable for parenteral use. Systemic
toxicity from application to skin or ulcerated areas is rare, but
it may cause allergic reactions and occasional anaphylaxis has
been described. It is found in many ointments and ophthalmic
preparations, usually together with other components,
including polymyxins, neomycin and corticosteroids.
Bacitracin is not absorbed by mouth but oral preparations
have been used for suppression of gut flora, including
C. difficile.
Mechanism of action
Bacitracin interferes with bacterial cell wall formation by inhibiting peptidoglycan synthesis, the major cell wall component in Gram positive bacteria. The lipid C-55-isoprenyl pyrophosphate (IPP) normally carries peptidoglycan units across the bacterial cell membrane. Upon delivery, the IPP is dephosphorylated by a membrane-associated pyrophosphatase to C-55-isoprenyl phosphate (IP). This enables the lipid to bind new cargo. Mediated by a metal ion, bacitracin forms a complex with IPP that inhibits its dephosphorylation to IP. Consequently, the amount of IP decreases, whereby the formation of the bacterial cell wall is hindered. Bacitracin is also thought to damage the bacterial cytoplasmic membrane. It may be either bactericidal or bacteriostatic, depending on the susceptibility of the infecting organism and local drug concentration.
Clinical Use
Bacitracin is mainly used topically for the treatment of skin, eye and ear infections, and the prevention of wound infections. It is especially employed for minor skin injuries, such as cuts, scrapes, or burns. Although it has been in use for almost half a century, it has only quite recently been recognized as a potent sensitizer, with occasional anaphylaxis. Thus, bacitracin should be used with caution. The value of bacitracin in the prevention of wound infections following clean surgical procedures is doubtful. A randomized, doubleblind study that evaluated approximately 1200 surgical wounds and compared bacitracin with white petrolatum for postoperative dressings demonstrated petrolatum to be equally as effective for postoperative wound care as bacitracin. The important advantage of white petrolatum over bacitracin was that its application led to a significantly lower rate of contact allergy. Other studies that have compared topical antimicrobial agents for use on clean surgical wounds have confirmed that such protocols do not improve healing or reduce the infection rate.
Clinical Use
Bacitracin is predominantly active against Gram-positive microorganisms, and parenteral use is limited to IM injection for infants with pneumonia and empyema caused by staphylococci resistant to other agents. It is rather neuro- and nephrotoxic and, therefore, is used in this manner with caution. Bacitracin also is widely employed topically to prevent infection in minor cuts, scrapes, and burns.
Toxicology
Nephrotoxicity (tubular and glomerular necrosis) and thrombophlebitis are the main toxic effects of bacitracin if it is administered systemically or intramuscularly. The renal toxicity of this drug may be largely due to the fact that it causes renal vasoconstriction. Nephrotoxicity usually does not occur in infants. Since the nephrotoxic effects may be additive, the concurrent or sequential use of systemic bacitracin with other nephrotoxic drugs should be avoided. Other adverse reactions include hypersensitivity reactions, anaphylaxis, hypotension, facial edema, urticaria, rash, diaphoresis, and blood dyscrasias, such as eosinophilia. When it is taken orally, it can induce anorexia, nausea, vomiting, and diarrhea. Cases of bacitracin-associated paresthesias, fever, and bone marrow toxicity, have also been described. Respiratory paralysis may occur in patients with a neuromuscular disease, such as myasthenia gravis. The use of bacitracin can result in overgrowth of nonsusceptible organisms, such as Candida spp. Bacitracin is classified as a Food and Drug Administration (FDA) pregnancy risk factor C agent, which means either that studies in animals have revealed adverse effects on the fetus (teratogenic, embryocidal, or other) or that there are no controlled studies in women, or that studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
Safety Profile
A poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Mutation data reported.
Potential Exposure
Bacitracin is used as an ingredient in antibiotic ointments to treat or prevent topical or eye infections. Commercial Bacitracin is a mixture of at least 9 bacitracins. Also used as a feed and drinking water additive for animals; as a food additive for human consumption.
First aid
In case of large-scale exposure, the directions formedicines (nonspecific, n.o.s.) would be applied as follows:Move victim to fresh air; call emergency medical care. Ifnot breathing, give artificial respiration. If breathing is diffi
storage
Color Code—Green: General storage may be used.
Shipping
UN 3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Purification Methods
Bacitracin has been purified by carrier displacement using n-heptanol, n-octanol and n-nonanol as carriers and 50% EtOH in 0.1 N HCl. The pure material gives one spot with RF ~0.5 on paper chromatography using AcOH:n-BuOH:H2O (4:1:5). [Porath Acta Chem Scand 6 1237 1952.] It has also been purified by ion-exchange chromatography. It is a white powder soluble in H2O and EtOH but insoluble in Et2O, CHCl3 and Me2CO. It is stable in acidic solution but unstable in base. It is a strong antibacterial. [Abraham & Bewton Biochem J 47 257 1950, Synthesis: Munekata et al. Bull Chem Soc Jpn 46 3187, 3835 1973, Beilstein 27 III/IV 5746.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Bacitracin Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29886 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8817 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 2993 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
View Lastest Price from Bacitracin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-02 | Bacitracin
1405-87-4
|
US $0.00-0.00 / Kg/Drum | 1KG | Bacitracin A≥40.0%;USP | 500KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-10-31 | Bacitracin
1405-87-4
|
US $30.00-42.00 / mg | 60IU/mg | 10g | TargetMol Chemicals Inc. | ||
 |
2024-10-31 | Bacitracin
1405-87-4
|
US $30.00-42.00 / mg | 60IU/mg | 10g | TargetMol Chemicals Inc. |
-

- Bacitracin
1405-87-4
- US $0.00-0.00 / Kg/Drum
- Bacitracin A≥40.0%;USP
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Bacitracin
1405-87-4
- US $30.00-42.00 / mg
- 60IU/mg
- TargetMol Chemicals Inc.
-

- Bacitracin
1405-87-4
- US $30.00-42.00 / mg
- 60IU/mg
- TargetMol Chemicals Inc.
1405-87-4(Bacitracin)Related Search:
1of4