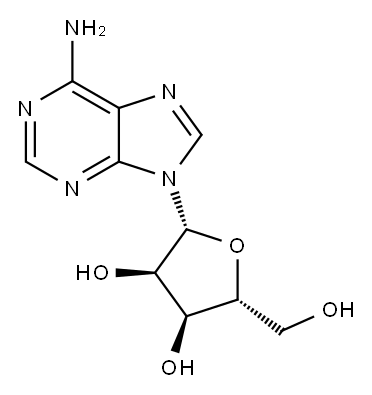
Adenosine 5'-monophosphate
- CAS No.
- 61-19-8
- Chemical Name:
- Adenosine 5'-monophosphate
- Synonyms
- AMP;VITAMIN B8;ADENOSINE PHOSPHATE;AMP.H2;adenylate;Adenosine Monophosphate (Amp);)-monophosphate disodium salt (mixed isomers);((2R,3S,4R,5R)-5-(6-aMino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)Methyl dihydrogen phosphate;a5mp;adenyl
- CBNumber:
- CB9206528
- Molecular Formula:
- C10H14N5O7P
- Molecular Weight:
- 347.22
- MDL Number:
- MFCD00005750
- MOL File:
- 61-19-8.mol
- MSDS File:
- SDS
| Melting point | 178-185 °C |
|---|---|
| Boiling point | 798.5±70.0 °C(Predicted) |
| Density | 2.32±0.1 g/cm3(Predicted) |
| FEMA | 4224 | ADENOSINE MONOPHOSPHATE; MONOSODIUM, OR DISODIUM ADENYLATE |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: with addition of mild alkalisoluble |
| form | Crystalline Powder |
| pka | 3.8, 6.2(at 25℃) |
| color | Colorless to white |
| PH | 3.84 |
| Water Solubility | Soluble in water. |
| λmax | 257 (pH 1);259 (pH 7) |
| Merck | 14,158 |
| Stability | Hygroscopic |
| InChI | InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 |
| InChIKey | UDMBCSSLTHHNCD-KQYNXXCUSA-N |
| SMILES | P(OC[C@H]1O[C@@H](N2C3C(=C(N=CN=3)N)N=C2)[C@H](O)[C@@H]1O)(O)(O)=O |
| CAS DataBase Reference | 61-19-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 415SHH325A |
| EPA Substance Registry System | 5'-Adenylic acid (61-19-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H335-H315 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AU7480500 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29389090 | |||||||||
| Hazardous Substances Data | 61-19-8(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 intraperitoneal in mouse: 4gm/kg | |||||||||
| NFPA 704 |
|
Adenosine 5'-monophosphate price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2340 | 5′-Adenylic Acid Pharmaceutical Secondary Standard; Certified Reference Material | 61-19-8 | 1G | $200 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1012178 | 5′-Adenylic acid | 61-19-8 | 500mg | $441 | 2024-03-01 | Buy |
| TCI Chemical | A0158 | 5'-Adenylic Acid >98.0%(HPLC)(T) | 61-19-8 | 1g | $19 | 2024-03-01 | Buy |
| TCI Chemical | A0158 | 5'-Adenylic Acid >98.0%(HPLC)(T) | 61-19-8 | 5g | $48 | 2024-03-01 | Buy |
| Alfa Aesar | L14051 | Adenosine-5'-monophosphoric acid, 99% (dry wt.), water <6% | 61-19-8 | 1g | $22.65 | 2024-03-01 | Buy |
Adenosine 5'-monophosphate Chemical Properties,Uses,Production
Chemical Properties
White crystalline powder. Melting point: 196-200°C (decomposition). Specific optical rotation:-47.5° (20°C, 2% in sodium hydroxide (2%)). Soluble in water, slightly soluble in alcohol, insoluble in ether.
Applications
Clinically it is used to treat disseminated sclerosis, porphyria, itching, liver disease, varicose ulcer complication. Eye drops with denosine monophosphate as the main component can be used to treat eye fatigue, central amphiblestitis, ocular pannus, herpes and other corneal surface diseases. Intramuscular injection shows local erythema, generalized essential telangiectasia, red face, dizziness, breathing difficulty and palpitation.
As nutrition enhancer; as intermediate to produce nucleotide drugs; as food additive; as biological products; as dietary supplement and biochemical reagent. It is used to produce adenosine triphosphate (ATP), cyclic adenylate (cAMP) and other biochemical drugs. It is a type of nucleotide products and to produce antiviral drugs, synthetic energy drugs and cardiovascular and cerebrovascular drugs, such as adenosine, ATP, 3 '-5'-cyclic adenosine monophosphate.
Preparation
Candida utilis fungi is treated with hot water to obtain nucleic acids, which is hydrolyzed by enzymes and separated to obtain the final product.
Take mycelia as the raw material:
Subtraction and sedimentation of mycelia are carried out with 3 times amount of water. Industry base is added to reach a base concentration of 0.25% (g/100mL). After stirring for 1 hours, 20% sulfuric acid is added until the PH value is 7. The solution is filtrated and the pH value of filtrate is adjusted with 20% sulfuric acid to 2.5. Centrifugation is then applied to obtain nucleic acid mud.
mycelia [sodium hydroxide]→[1h] extract solution [20% sulfuric acid] → [pH=7, filtration] filtrate [20% sulfuric acid] →[pH=2.5, centrifugation] nucleic acid mud
1% nucleic acid solution is made by subsequent dissolution, enzymolysis, absorption and elution. 10% ammonia water is then used to adjust the pH value to 6-6.2. Then the mixture is heated at 90°C for 20 min and cooled. After centrifugation, the supernatant is heated up to 65-70°C and added with 1/3 phosphodiesterase solution. After 2 hours, the temperature is raised to 90°C. 10min later, it is cooled to room temperature. 20% sulfuric acid is added to adjust the pH value to 2.5-3. After filtration, the filtrate is adjusted with ammonia solution until the pH=7.2-7.5. 0.3% (3 g/L) diatomite is then added to assist the filtration. The corresponding supernatant is filtered with an anion exchange resin column (717-type) and eluted with 0.05 mol/L sulfuric acid. Afterwards, absorption, elution and crystallization are carried out. The detailed procedures of these three steps are:
The eluate is first absorbed with a cation exchange column (732-type) and eluted with distilled water (pH=1.5). The collection of eluate starts when the eluate turned red with bromine water. The eluate is then concentrated to 80-90 mg/mL under reduced pressure, added with diatomite and stirred for 30 min before filtration. The filtrate is adjusted with 6 mol/L hydrochloric acid till pH=2.5. To realize full crystallization, the filtrate is cooled during stirring. After followed filtration, it is washed with dry ethanol for 3 times. Vacuum drying at 60°C is applied to obtain the final AMP products.
Nucleic acid mud [ammonia gas] →[pH=6-6.2, 90°C, 20min] supernatant [phosphodiesterase] →[65-70°C,2h,] hydrolysis solution [717 resin] →absorbent [sulfuric acid] →elution solution [732 resin] →AMP, CMP, GMP absorbent mixtures.
Usage restriction
GB 2760-2001:infant formula milk powder 0.2~0.58 g/kg (based on the total amount of nucleotide)
Category
Toxic substance
Toxicity Grading
Middle toxicity
Acute Toxicity
Peritoneal-mouse LD50: 4000 mg/kg
Flammability Hazardous Characteristics
Flammable; generation of toxic nitrogen oxides and phosphorous oxide smoke when heated.
Storage and Transport
Stored in well ventilated area, low temperature, and dry.
Extinguishing Agent
Dry powder, foam, sand, carbon dioxide.
Description
Adenosine-5'-monophosphoric acid is a nucleotide that is synthesized from adenosine triphosphate and inosine monophosphate. Adenosine-5'-monophosphoric acid is an important molecule in the body because it is a substrate for cyclic AMP, which regulates many cellular processes. Adenosine-5'-monophosphoric acid also has biochemical properties that are similar to those of the neurotransmitter serotonin and it can activate the 5-HT2 receptors.
Chemical Properties
colorless to white crystalline powder
Uses
Adenosine 5'-Monophosphate is a natural occurring nucleotide and a useful ligand determinant that facilitate the binding of APS reductase inhibitors and activates adenosine receptor agonists.
Uses
A useful ligand determinant that facilitates the binding of APS reductase inhibitors.
Uses
vasodilator, neuromodulator
Uses
Adenosine 5'-monophosphate is a nucleotide (building blocks of nucleic acid) added to skin care products to bind water and moisture.
Uses
antiasthmatic
Definition
ChEBI: A purine ribonucleoside 5'-monophosphate having adenine as the nucleobase.
brand name
Adenyl (Wyeth-Ayerst); My-BDen (Bayer.
Biological Activity
adenosine 5'-monophosphate is an ester of phosphoric acid with the nucleoside adenosine.
Safety Profile
Slightly toxic by intraperitoneal route. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of PO, and NOx.
Adenosine 5'-monophosphate Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou Weitai Biological Pharmaceutical Co.,Ltd. | +8613282012786 | sales@weitai-pharmaceutical.com | China | 61 | 58 |
| Wuhu Nuowei chemistry Co., Ltd. | +86-0553-2911116-802 +86-18055311600 | sales1@nuowei-chem.com | China | 1638 | 58 |
| Hangzhou Measure Life Technology Co., LTD | +8613343730176 | 2924244016@qq.com | China | 83 | 58 |
| Hangzhou Meiya Pharmaceutical Co Ltd | +86-021-33686691 +8618001850931 | sales@meiyapharm.com | China | 58 | 58 |
| Wuhan Fortuna Chemical Co.,Ltd | +8618007136271 | hk@fortunachem.com | China | 5992 | 58 |
| Xinxiang Aurora Biotechnology Co.,Ltd. | +86-0086-0373-3088722 +8618637352520 | info@aurora-biotech.com | China | 97 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12809 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5868 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
Related articles
- Adenosine 5'-Monophosphate: Physiological Role and Applications in Asthma Management
- Adenosine 5′-monophosphate is a potent pharmacological agent increasingly used in indirect bronchial challenge tests in patien....
- Oct 21,2024
Related Qustion
- Q:What is the effect of injection of Adenosine 5'-monophosphate on the body temperature of mammals?
- A:Adenosine 5'-monophosphate (5'-AMP), as an adenosine A1 receptor agonist, can induce a decrease in body temperature in mammals....
- Dec 16,2024
View Lastest Price from Adenosine 5'-monophosphate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-24 | Adenosine 5'-monophosphate
61-19-8
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%min HPLC | 1000 KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-04-24 | Adenosine 5'-monophosphate
61-19-8
|
US $0.00-0.00 / Kg | 1Kg | 98% | 20Ton | Xinxiang Aurora Biotechnology Co.,Ltd. | |
 |
2025-04-24 | Adenosine 5’-monophosphate(AMP-H)
61-19-8
|
US $0.00-0.00 / kg | 1kg | ≥98% | 25MT | Hangzhou Meiya Pharmaceutical Co Ltd |
-

- Adenosine 5'-monophosphate
61-19-8
- US $0.00-0.00 / Kg/Drum
- 98%min HPLC
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Adenosine 5'-monophosphate
61-19-8
- US $0.00-0.00 / Kg
- 98%
- Xinxiang Aurora Biotechnology Co.,Ltd.
-

- Adenosine 5’-monophosphate(AMP-H)
61-19-8
- US $0.00-0.00 / kg
- ≥98%
- Hangzhou Meiya Pharmaceutical Co Ltd