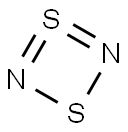
Disulfur dinitride
- CAS No.
- 25474-92-4
- Chemical Name:
- Disulfur dinitride
- Synonyms
- disulfur dinitride;1λ4δ2-1,3,2,4-Dithiadiazete;1l4d2-1,3,2,4-Dithiadiazete
- CBNumber:
- CB93209357
- Molecular Formula:
- N2S2
Lewis structure

- Molecular Weight:
- 92.1434
- MDL Number:
- MOL File:
- 25474-92-4.mol
Disulfur dinitride Chemical Properties,Uses,Production
Chemical Properties
Well-formed, colorless crystals; very volatile; unpleasant iodinelike odor; stable only at low temperature; becomes dark after a short exposure to 20°C; sublimes at 10-2 mm. even at room temperature; polymerizes readily to (SN)X; in the presence of traces of moisture, about 67% of the S2N2 polymerizes to (SN)x, while 33% dimerizes to S4N4; detoned by shock, friction and temperature above 30°C; soluble in alcohols, yielding yellowish red solutions; readily forms colorless solutions in benzene, ether, carbon tetrachloride, acetone, tetrahydrofuran, dioxane; in the absence of moisture, the colorless solutions are more stable than the solvent-free substance (however, the addition of traces of alkali metals, some NaOH, KCN or Na2CO3 causes instantaneous and complete dimerization); crystals are not wetted by water and acids (for this reason, hydrolysis with these solvents occurs very slowly); vigorous reaction with alkali solutions; dissolves rapidly in 2 N NaOH, giving a yellow solution, with larger crystals becoming black and detonating, giving off a pungent gas.
Production Methods
Disulfur dinitride is prepared by thermal degradation of S4N4. A small amount (1-2 g) of S4N4 is placed in the reactor. A 7-cm-long part of the section heated by the upper furnace is filled with tightly compressed silver wool. The apparatus is evacuated to 0.005 mm. The first condensation trap is cooled with dry ice methanol, and the second with liquid nitrogen. The upper furnace is heated to 300°C, and only then is the lower one switched on. The cold finger soon becomes coated with a blue film, and colourless to faintly yellow crystals form in the inlet tube to the first trap. After 6-8 hours, the thermal degradation of S4N4 is complete. The furnaces and trap coolants are removed, and the apparatus is flushed with dry air or N2.
Definition
ChEBI: Disulfur dinitride is an inorganic heterocyclic compound with formula N2S2 and consisting of alternating sulfur and nitrogen atoms making up a 4-membered ring structure that is virtually square and planar. It is shock-sensitive and decomposes explosively above 30℃. It is an inorganic heterocyclic compound, a sulfur molecular entity and a nitride.
Purification Methods
The light-grey crystalline coating in the first trap (which is reddish, with a blue rim, where it extends beyond the cooling zone) is extracted several times with 10 ml portions of dry ether until only a few dark-blue or shiny metallic crystals remain. The first extract is a deep red because of the byproduct S4N2; since S4N2 dissolves readily, the colour of the other extracts is lighter. The combined ether solution is filtered and placed in a conical ground glass flask (which narrows to a point at the bottom), provided with an adapter permitting reverse filtration with the exclusion of moisture. The flask is then cooled to —80°C in a Dry Ice methanol bath; the white S3NS crystals precipitate. These are separated from the ether by reverse filtration. To purify the S3N3, it can be sublimed at room temperature in a high vacuum. Beautiful, large, colourless crystals are obtained. The yield, prior to sublimation, is 80%.
Disulfur dinitride Preparation Products And Raw materials
Raw materials
Preparation Products
Disulfur dinitride Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| Supplier | Advantage |
|---|---|
| Shaanxi Didu New Materials Co. Ltd | 58 |