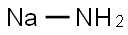Sodium amide
- CAS No.
- 7782-92-5
- Chemical Name:
- Sodium amide
- Synonyms
- SODAMIDE;NaNH2;m amide;Natriumamid;Aminosodium;SODIUM AMIDE;Sodium amino;Sodiumamide,94%;SODIUM AMIDE 95%;Sodium amide (8CI)
- CBNumber:
- CB9362037
- Molecular Formula:
- H2NNa
Lewis structure

- Molecular Weight:
- 39.01
- MDL Number:
- MFCD00011117
- MOL File:
- 7782-92-5.mol
- MSDS File:
- SDS
| Melting point | 210 °C (lit.) |
|---|---|
| Boiling point | 400 °C (lit.) |
| Density | 1.39 g/cm3 (25℃) |
| Flash point | 85 °F |
| storage temp. | Store below +30°C. |
| solubility | reacts with H2O |
| form | crystalline |
| color | Greyish white powder |
| Odor | Ammonia like |
| Water Solubility | REACTS VERY VIOLENTLY, EVEN EXPLOSIVELY |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,8576 |
| Stability | Stability Flammable. Reacts violently with water producing very toxic fumes. In case of fire do not use water, but instead smother with soda ash. May form explosive peroxides if heated, or if stored for extended periods in contact with air or oxygen. Incompatible with water and aqueous solutions, carbon dioxide, halogenated hydrocarbo |
| CAS DataBase Reference | 7782-92-5(CAS DataBase Reference) |
| FDA UNII | 5DB3G6PX9D |
| EPA Substance Registry System | Sodium amide (Na(NH2)) (7782-92-5) |
| UNSPSC Code | 26111700 |
| NACRES | NA.23 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H225-H261-H304-H314-H336-H361d-H373-H412 |
| Precautionary statements | P210-P231+P232-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | F,C,Xi,N |
| Risk Statements | 14/15-19-34-20/21-10-67-65-63-48/20-11-36/37-15/29-14-50-29 |
| Safety Statements | 26-43-45-62-7/8-43D-36/37/39-27-16-61 |
| RIDADR | UN 3129 4.3/PG 2 |
| WGK Germany | 2 |
| F | 3-10-23 |
| Autoignition Temperature | 450 °C |
| TSCA | Yes |
| HS Code | 2853 90 90 |
| HazardClass | 4.3 |
| PackingGroup | II |
| Hazardous Substances Data | 7782-92-5(Hazardous Substances Data) |
Sodium amide price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.14095 | Sodium amide crystalline for synthesis | 7782-92-5 | 100g | $66.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.14095 | Sodium amide crystalline for synthesis | 7782-92-5 | 1kg | $308 | 2024-03-01 | Buy |
| Sigma-Aldrich | 208329 | Sodium amide 98% | 7782-92-5 | 50g | $59.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 208329 | Sodium amide 98% | 7782-92-5 | 1kg | $138 | 2024-03-01 | Buy |
| Sigma-Aldrich | 432504 | Sodium amide 95% | 7782-92-5 | 25g | $52.5 | 2024-03-01 | Buy |
Sodium amide Chemical Properties,Uses,Production
Chemical properties and main uses
Sodium amide is white or olive green crystalline powder, it has ammonia odor, chemical formula is NaNH2, the molecular weight is 39.01, melting point is 210℃, boiling point is 400℃. When heated to 500~600℃, it can decompose into sodium, nitrogen, and hydrogen. It can react violently with water, and generates sodium hydroxide, and releases ammonia. It is slightly soluble in liquid ammonia, it reacts slowly with alcohol. It quickly absorbs carbon dioxide and water in the air, it should be sealed. Magnesium, zinc, molybdenum, tungsten and quartz, glass, silicate and other liquid substances are soluble in liquid state sodium amide.-1 Monovalent radicals-amino NH-2 ionic compounds is formed by sodium ions and ammonia molecules which gets rid of hydrogen atom. Sodium amide is flammable, explosive, corrosive and deliquescence. Since the amino group has lone pair of electrons, it is easy to combine protons, so intense hydrolysis happen when meets water, the solution is alkaline: NaNH2 + H2O = NaOH + NH3. It is dissolved in hot ethanol and liquid ammonia. Dust is toxic. It has severe irritation for skin, eyes and respiratory system. After touch the skin, it can cause corrosive burns. It can be used in organic synthesis aminating agent; condensation accelerator; dehydrating agent, dehalogenating agent; polymerization initiator and manufacturing hydrazine, sodium cyanide, azide, cyanide, hydrazine, indigo raw materials.
Sodium amide is readily oxidized in air to form a layer of yellow variety of oxidation products on the surface. The partial oxidation product is explosive, explosion can happen with heat or friction. When the sodium amide is heated to 300~330℃ in vacuo, it can decompose into nitrogen, sodium, hydrogen and ammonia. It should be filled with inert atmosphere in the bottle sealed and stored to avoid contacting with air, water or fire to avoid explosion and fire. When sodium amide reacts with carbon monoxide, sodium cyanide can generate, this reaction can be used for synthesis of sodium cyanide. Sodium amide is generally used for organic synthesis condensation promoting agent, dehydrating agent, alkylating agent. With the reaction of molten sodium with gaseous ammonia at 300~400 ℃, sodium amide can be obtained industrially, sodium amide is obtained by the reaction of sodium and ammonia in the presence of catalyst at room temperature in laboratory.
Toxicity
Dust is toxic, it can cause corrosive burns, it can severely irritate the eye, skin and respiratory system.
People should wear gas masks and gloves when disposes of escaping material, it should be mixed with dry sand and sent to open area, after reacting with plenty of water, it can be sent to the wastewater system. Pollution ground should also be rinsed with water; when contacts with skin, eye irritation, people should immediately wash with water; when strays into the mouth, people should immediately gargle, drink water and vinegar or 1% acetic acid, and then be sent to hospital for treatment.
Uses
1. It can be used as the condensation accelerator in organic chemical reactions. It is raw material in synthesis of vitamin A. It can be also used as dehydrating agent, dehalogenating agent, alkylating agent, ammoniated agent. In liquid ammonia, the dissociation of NHf in liquid ammonia can be used as initiator of anionic polymerization producting polyvinyl chloride ion. It is also used in the manufacture azides, cyanide, hydrazine, and indigo.
2. It can be used as condensation-accelerating agent in organic synthesis, dehydration and the like.
The above information is edited by the chemicalbook of Wang Xiaodong.
Production method
High-temperature method: sodium metal is melt at 97~100℃, then slowly passes through dehydration liquid ammonia, when be heated to 350 ~360℃, sodium amide and hydrogen can generate, the reaction is cooled, cemented sheet, and then pulverized to obtain sodium amino finished.
2Na + 2NH3 → 2NaNH2 + H2 ↑
Category
When meets water, it is combustible goods.
Explosive hazardous characteristics
It can intensely react with water to produce combustible gas, maybe it could explode.
Flammability hazard characteristics
It can decompose into products of toxic nitrogen oxides; flammable and explosive hydrogen gas and caustic sodium hydroxide when meets water.
Storage characteristics
Treasury ventilation low-temperature drying; it should be stored separately with acid, alkali ; it should be moistureproof.
Extinguishing agent
Dry sand, carbon dioxide.
Chemical Properties
grey powder
Physical properties
White crystalline powder with odor of ammonia; orthogonal crystals; density 1.39 g/cm3; melts at 210°C; begins to volatilize at 400°C; decomposes at 500°C; decomposed by water and hot alcohol; in fused state it dissolves zinc, magnesium and other metals, as well as, quartz, glass, and silicates.
Uses
Reagent for: Synthesis of allylic amines and amides1 Synthesis of antibacterials2 Aggregative activation and heterocyclic chemistry3 Phenylation with diphenyliodonium chloride4
Uses
Sodium Amide is widely used in organic chemical synthesis as a strong base in reactions such as in the preparation of phenylacetylene from 1,2-dibromophenylethane. Also used in the synthesis of 2,6-diamination of substituted pyridines as initiator of heterogeneous chichibabin reaction.
Uses
Dehydrating agent. In the production of indigo and hydrazine. Intermediate in the preparation of sodium cyanide. In ammonolysis reactions, in Claisen condensations, alkylation of nitriles and ketones, synthesis of ethynyl Compounds, acetylenic carbinols. Fused NaNH2 dissolves metallic Mg, Zn, Mo, W, quartz, glass, silicates and other substances.
Preparation
Sodium amide is prepared by passing dry ammonia gas over sodium metal at 350°C: 2Na + 2NH3 → 2NaNH2 + H2Also, it may be prepared by reacting sodium metal with liquid ammonia in the presence of a catalyst such as iron(III) nitrate. The compound must be stored in well-sealed containers free from air or moisture.
Definition
A white ionic solid formed by passing dry ammonia over sodium at 300–400°C. The compound reacts with water to give sodium hydroxide and ammonia. It reacts with red-hot carbon to form sodium cyanide and with nitrogen(I) oxide to form sodium azide. Sodamide is used in the Castner process and in the explosives industry.
Reactions
Sodamide, sodamine, NaNH2, white solid, formed by reaction of sodium metal and dry NH3 gas at 350 °C (662 °F), or by solution of the metal in liquid ammonia. Reacts with carbon upon heating, to form sodium cyanide, and with nitrous oxide to form sodium azide, NaN3.
General Description
Odorless colorless solid. Denser than water.
Air & Water Reactions
Highly flammable. Reacts violently with water and bursts into flame.
Reactivity Profile
Sodium amide is a powerful reducing agent. Reacts violently with oxidizing agents. Reacts violently with steam and water to form caustic NaOH and NH3 vapors [Bergstrom et al., Chem. Reviews, 12:6 1932]. May form explosive compounds in the presence of water and carbon dioxide [Handling Chemicals Safely 1980 p 826]. Liable to deflagration upon heating and friction. Forms an explosive peroxide on storage. When Sodium amide and chromic anhydride are mixed together a vigorous reaction results; the same occurs with other oxidizing agents including dinitrogen tetraoxide, potassium chlorate, sodium nitrite. [Mellor 11:234 1946-47]. Reaction with 1,1-diethoxy-2- chloroethane produces sodium ethoxyacetylide, which is extremely pyrophoric [Rutledge 1968 p. 35]. Reactions with halogenated hydrocarbons may be violently explosive. Sodium amide forms toxic and flammable H2S gas with CS2. (714).
Hazard
Flammable, dangerous fire risk.
Health Hazard
Ammonia gas formed by reaction of solid with moisture irritates eyes and skin. Solid causes caustic burns of eyes and skin. Ingestion burns mouth and stomach in same way as caustic soda and may cause perforation of tissue.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating ammonia gas may be formed.
Safety Profile
An intense irritant to tissue, skin, and eyes. Flammable by chemical reaction. Ignites or explodes with heat or grinding. Explosive reaction with moisture, chromium trioxide, potassium chlorate, halocarbons (e.g., 1,l -diethoxy-2chloroethane), oxidants, sodium nitrite, air. Can become explosive in storage. Violent reaction with dinitrogen tetraoxide. Will react with water or steam to produce heat and toxic and corrosive fumes of sodium hydroxide and ammonia. When heated to decomposition it emits highly toxic fumes of NH3 and Na2O. See also AMIDES.
Purification Methods
It reacts violently with H2O and is soluble in liquid NH3 (1% at 20o). It should be stored in wax-sealed containers in small batches. It is very hygroscopic and absorbs CO2 and H2O. If the solid is discoloured by being yellow or brown in colour, then it should be destroyed as it can be highly EXPLOSIVE. It should be replaced if discoloured. It is best destroyed by covering it with much toluene and slowly adding dilute EtOH with stirring until all the ammonia is liberated (FUME CUPBOARD). [Dennis & Bourne Inorg Synth I 74 1939, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 465 1963, Bergstrom Org Synth Coll Vol III 778 1955.]
Sodium amide Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12814 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1803 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1001 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5906 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| Xingtai Haoxun Import and Export Trade Co., Ltd. | +8617733976525 | lisa@xthaoxun.com | CHINA | 249 | 58 |
View Lastest Price from Sodium amide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-03-31 | Sodium amide
7782-92-5
|
US $10.00 / KG | 100KG | 99% | 100 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-03-21 | Sodium amide
7782-92-5
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd | |
 |
2025-03-21 | Sodium amide
7782-92-5
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd |
-

- Sodium amide
7782-92-5
- US $10.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Sodium amide
7782-92-5
- US $0.00 / KG
- 99%
- Hebei Mujin Biotechnology Co.,Ltd
-

- Sodium amide
7782-92-5
- US $0.00 / KG
- 99%
- Hebei Mujin Biotechnology Co.,Ltd