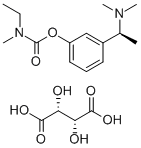
Rivastigmine tartrate
- CAS No.
- 129101-54-8
- Chemical Name:
- Rivastigmine tartrate
- Synonyms
- CS-118;ENA-713;SDZ-ENA 713;Rivastigmine tartrate;Rivastigimine tartrate;RivastigMine L-Tartrate;RivastigMine Bitartrate;S-Rivastigmine tartrate;rivastigMine ditartrate;Rivastigmine-d6 tartarate
- CBNumber:
- CB9500851
- Molecular Formula:
- C18H28N2O8
- Molecular Weight:
- 400.42
- MDL Number:
- MFCD03700731
- MOL File:
- 129101-54-8.mol
- MSDS File:
- SDS
| Melting point | 123-1250C |
|---|---|
| alpha | D20 +4.7° (c = 5 in ethanol) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble15mg/mL, clear |
| form | powder |
| color | white to beige |
| InChI | InChI=1/C14H22N2O2.C4H6O6/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4;5-1(3(7)8)2(6)4(9)10/h7-11H,6H2,1-5H3;1-2,5-6H,(H,7,8)(H,9,10)/t11-;1-,2-/s3 |
| InChIKey | GWHQHAUAXRMMOT-AAUFCPPMNA-N |
| SMILES | C1(=CC=CC([C@H](C)N(C)C)=C1)OC(=O)N(C)CC.[C@H](O)(C(=O)O)[C@@H](O)C(=O)O |&1:5,18,23,r| |
| CAS DataBase Reference | 129101-54-8(CAS DataBase Reference) |
| FDA UNII | 9IY2357JPE |
| NCI Drug Dictionary | rivastigmine tartrate |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H411 | |||||||||
| Precautionary statements | P264-P270-P273-P301+P310-P391-P405 | |||||||||
| RIDADR | UN 2811 6.1 / PGII | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FA9550000 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29242990 | |||||||||
| NFPA 704 |
|
Rivastigmine tartrate price More Price(52)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0881 | Rivastigmine tartrate ≥98% (HPLC) | 129101-54-8 | 50mg | $147 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1604858 | Rivastigmine tartrate United States Pharmacopeia (USP) Reference Standard | 129101-54-8 | 200mg | $472 | 2024-03-01 | Buy |
| TCI Chemical | R0093 | Rivastigmine L-Tartrate >98.0%(HPLC)(T) | 129101-54-8 | 1g | $186 | 2024-03-01 | Buy |
| TCI Chemical | R0093 | Rivastigmine L-Tartrate >98.0%(HPLC)(T) | 129101-54-8 | 5g | $644 | 2024-03-01 | Buy |
| Cayman Chemical | 14270 | Rivastigmine (tartrate) ≥95% | 129101-54-8 | 100mg | $117 | 2024-03-01 | Buy |
Rivastigmine tartrate Chemical Properties,Uses,Production
Pharmacological effects
Rivastigmine tartrate is rivastigmine Alzheimer's disease drugs, rivastigmine is physostigmine derivative by Novartis for the first time successfully developed the trade name Exelon (exelon), a molecule with there carbamate structure, is a kind of amino acid of selective cerebral cholinesterase inhibitor, can also inhibit the acetylcholinesterase and butyrylcholinesterase, cholinergic neurons by delaying the release of acetylcholine degradation and energy promoting cholinergic nerve conduction, can improve cognitive function disorders mediated by cholinergic, thereby improving the cognitive function of Alzheimer's disease patients. Rivastigmine plasma protein binding is weak, easily through the blood-brain barrier, which has a high degree of brain selectivity. It not only selectively acts on the most easily affected cerebral cortex and hippocampus, but also preferentially inhibits the dominant subtype of AChE in the brain, resulting in a reduction in the efficacy of peripheral cholinergic side effects. Rivastigmine in vivo half-life is short and long duration of action. Unlike tacrine, this product in the hippocampus and cortex of G1 enzyme inhibitory effect is stronger. Clinic for the treatment of mild to moderate Alzheimer-type dementia, or Alzheimer's disease can be suspected Alzheimer's disease clinically.
Alzheimerdisease
Alzheimer's disease (Alzheimerdisease, AD) is a kind of onset hidden attack the development of dementia, clinical with memory disorders, aphasia, apraxia, agnosia, executive function and cognitive impairment features. At the same time, that is accompanied by the psychological and behavioral abnormalities and obvious function of social life decline. AD duration course is 3 to 7 years, after the onset of a small number of patients can survive for more than 10 years or longer. In 1906, German neurologist Alzheimer first reported one case of 51-year-old female patient, brain autopsy found that age spots, he added that the disease has been reported neurofibrillary changes. Kraepelin (1913) would be named the disease of Alzheimer's disease. Thought that the disease is only found in pre-elderly, it is also known as Alzheimer's (preseniledementia). Later found brain senile dementia (seniledementia) and even the health of the elderly also have the same AD pathology, only a difference in degree. In the 1970s, the US National Institute of Gerontology Research Institute (NIA), the National neurology, language barriers and Stroke Research Institute (NINCDS) and the National Institute of Mental Health (NIMH) unified on senile dementia and AD mutual understanding of the relationship, in addition to age sooner than thought, both clinical symptoms and pathological brain changes were not significantly different, the Department of the same disease. Although the incidence of pre-elderly has been proposed, said AD2 type, is equivalent to the previous Alzheimer's disease, old age of AD1 type, the equivalent to senile dementia, but in the new diagnostic classification of literature in general, the onset before the age of 65 said early-onset AD, after 65 years, the onset of said late onset AD; in families predisposed called familial AD (FAD), said that no family predisposed sporadic AD.
Pharmacokinetics
Absorption: Rivastigmine completely absorbed quickly. About 1 hour to reach the peak plasma concentration. Because interacts with the target enzyme, biological drugs increase using more than the increased dose is expected values of about 1.5 times. The absolute bioavailability of taking 3 mg is about 36%. Rivastigmine with food can be allowed to absorb Tmax an extra 90 minutes to reduce the Cmax, AUC increased nearly 30%.
Distribution: the binding capacity of Rivastigmine with plasma protein is weak (approximately 40%). It easily passes the blood-brain barrier, surface volume of distribution is 1.8~2.7L/kg.
Metabolism: Rivastigmine mainly goes through cholinesterase mediated hydrolysis and rapid, extensive metabolism (plasma half-life of about 1 hour). In vitro experiment results show that this metabolite only a weak cholinesterase inhibition (<10%). In vitro and animal experimental results show that the majority of the cytochrome P450 isoenzymes are rarely involved in rivastigmine hydrogen tartrate metabolism. In vivo experiments also confirmed the bitartrate.
Excretion: In urine, it is not found rivastigmine hydrogen tartrate prototype drug. The main metabolites excreted through the kidneys. 14C isotope labeled rivastigmine hydrogen tartrate administered within 24 hours, most rapidly excreted by the kidneys (> 90%) and only <1% of the drug excreted in the faeces. Alzheimer's disease patients have no body weight Rivastigmine or a metabolite accumulation.
Elderly subjects: Despite the elderly Rivastigmine biological utilization degree is higher than that of healthy young volunteers, but after 50 to 92 years in patients with Alzheimer test results showed that the bioavailability does not change with increasing age.
Reference: Yuanyong Gui, Tang Yong editor psychiatric outpatient and emergency manual Nanjing: Jiangsu Science and Technology Publishing House .2006.
Indications
Used to treat the symptoms of mild to moderate Alzheimer's disease dementia symptoms.
Side effects
The most commonly reported adverse drug reactions were gastrointestinal reactions,, including nausea (38%), vomiting (23%), especially in the dosage of the more obvious. In clinical trials,it found that women were more prone to gastrointestinal reactions and weight loss.
The following table lists the adverse reactions of this product, including clinical trials and post-marketing reports of all cumulative.
Adverse reactions Frequency of occurrence
Cardiac abnormalities
Arrhythmia Very rare
Angina rare
Vascular abnormalities
hypertension Very rare
Gastrointestinal system disorder
nausea Very common
Vomit Very common
Loss of appetite Very common
diarrhea Very common
Abdominal pain and indigestion common
Gastric and duodenal ulcers rare
Gastrointestinal bleeding Very rare
Mild pancreatitis Very rare
Skin and subcutaneous tissue abnormalities
Increased sweating common
rash rare
General exception
Fatigue and weakness common
Unwell common
Accidental fall Occasionally
survey
Weight loss common
Above Pictured: The common adverse reactions of Rivastigmine in various body systems table.
The frequency of adverse reactions were follows conversion: very common (>=1/10), common (>=1/100, <1/10), occasionally (>=1/1 000, <1/100), rare (>=1/10000, <1/1 000), very rare (<1/10000), including case reports.
In addition, the following side effects in patients taking Exelon in patients with the occurrence rate of at least a given placebo 2%, increased sweating, general malaise, weight loss, tremor. Female patients with nausea, vomiting, loss of appetite and weight loss are more sensitive. Exelon does not cause changes in any laboratory examinations, including liver function, or electrocardiogram, and there is no need special care.
There is a case of patients taking this product, while the joint use of several other drugs, the emergence of Stevense-Johnson syndrome.
In the initial phase of treatment, the incidence of adverse reactions is higher than the maintenance phase. In case of severe intolerable (such as severe nausea, vomiting, etc.), you should consider taking 3 times a day.
Pregnant and lactating women
Pregnancy: Animal experiments show that rivastigmine has no teratogenic effects. Because the lack of experimental data security using this service when people pregnancy, so pregnant women using this service should be weighed the pros and cons.
Lactation: whether the product is secreted from human milk, which is unclear. However, in animals rivastigmine tartrate can be secreted from the milk. Patients using this service should stop breastfeeding. Accordingly, patients with ongoing breast feeding should not be using this service.
Pediatric
Children are not recommended to take this product.
Elderly patients
Although the elderly’s rivastigmine bitartrate bioavailability is higher than that of young healthy volunteers, but after 50 to 92 years in patients with Alzheimer test results show that rivastigmine tartrate bioavailability does not change with increasing age.
Drug Interactions
(1) Rivastigmine is mainly through hydrolysis cholinesterase metabolism, cytochrome P450 isozymes are rarely involved in its metabolism. Therefore, the product and by other drugs metabolized by these enzymes does not exist pharmacokinetic interactions.
(2) study in healthy volunteers found the product (a single dose of 3mg) with digoxin, warfarin, diazepam or fluoxetine, have no pharmacokinetic interactions. Warfarin induced by prothrombin time extension are not affected by this product. After combination of digoxin and the product found no adverse impact on cardiac conduction.
(3) In clinical studies of patients with Alzheimer's disease (AD), the goods and some commonly used prescription drugs combined, such as antacids, antiemetics, hypoglycemic agents, β-blockers, calcium channel blockers agents, inotropic agents, anti-angina drugs, non-steroidal anti-inflammatory drugs, estrogens, analgesics, tranquilizers, antihistamines, etc., and risk of clinically relevant adverse reaction does not increase
(4) In view of the pharmacokinetic effect of this product, this product should not be used in combination with other cholinergic drugs, it may interfere with the activity of anticholinergic drugs.
(5) There is no experience of this product with anti Parkinson drugs, anti anxiety drugs (except for stability), antipsychotic drugs, antiepileptic drugs, antidepressant drugs.
(6) rivastigmine tartrate as a cholinesterase inhibitor, during anesthesia, it can be used to enhance succinylcholine-type muscle relaxant effect.
Contraindications
It is known for rivastigmine tartrate, other carbamate derivatives or other components of the formulation in patients allergic to this ban. Severe liver failure patients with severe Rivastigmine efficacy and safety have not been studied.
Precautions
1. As inhibitors of acetylcholinesterase, rivastigmine tartrate can improve succinylcholine-type muscle relaxant effect. Therefore, before anesthesia should have the right to stop taking this interim period. The goods and other cholinergic or anti-cholinergic agents in combination, should be particularly cautious (see Drug Interactions).
2. Due to their pharmacological effects, cholinesterase inhibitors on heart rate may produce vagal nervous effects. Like with other cholinergic drugs, when administered to sick sinus syndrome or other heart block in patients using this service, you must be more cautious (see Adverse reactions).
3. cholinergic nerve excitability can cause gastric acid secretion. Although no evidence of corresponding symptoms worsen during the clinical trials, there is a high risk of gastric ulcer patients, such as those with a history of ulcer disease, or accept non-steroidal anti-inflammatory drugs in patients with concomitant therapy should be used with caution.
4. Like other cholinesterase inhibitors, patients with a history of asthma or other obstructive pulmonary disease should be used with caution to rivastigmine bitartrate.
Chemical Properties
White to Off-White Powder
Uses
Nootropic & Alzheimer’s treatment
Uses
Rivastigmine Tartrate, a cholinesterase inhibitor with IC50 of 5.5 μM, ues as a parasympathomimetic or cholinergic agent for the treatment of mild to moderate Alzheimer disease.
Uses
A brain selective acetylcholinesterase inhibitor. Nootropic.
Definition
ChEBI: A tartrate salt obtained by reaction of rivastigmine with one equivalent of (R,R)-tartaric acid. A reversible cholinesterase inhibitor.
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biochem/physiol Actions
Rivastigmine is an orally available, brain penetrant, reversible cholinesterase inhibitor that enhances cognitive function in patients with Alzheimer′s and Parkinson′s diseases. Rivastigmine inhibits both butyrylcholinesterase and acetylcholinesterase.
storage
Store at RT
Rivastigmine tartrate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Mediking Biopharm Co., Ltd | +undefined15901403431 | sales01@mediking.cn | China | 150 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659 +86-13153181156 | sales@sdperfect.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Cangzhou Kangrui Pharma Tech Co. Ltd., | +86-18632776803 +86-13833998158 | cangzhoukangrui@126.com | China | 736 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
View Lastest Price from Rivastigmine tartrate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Rivastigmine tartrate
129101-54-8
|
US $0.00-0.00 / KG | 1KG | 98% HPLC | 1000kg | shandong perfect biotechnology co.ltd | |
 |
2024-11-15 | Rivastigmine tartrate
129101-54-8
|
US $55.00-47.00 / mg | 99.66% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-08 | Rivastigmine tartrate
129101-54-8
|
US $0.00-0.00 / g | 100g | 99% | 5kg | Cangzhou Kangrui Pharma Tech Co. Ltd., |
-

- Rivastigmine tartrate
129101-54-8
- US $0.00-0.00 / KG
- 98% HPLC
- shandong perfect biotechnology co.ltd
-

- Rivastigmine tartrate
129101-54-8
- US $55.00-47.00 / mg
- 99.66%
- TargetMol Chemicals Inc.
-

- Rivastigmine tartrate
129101-54-8
- US $0.00-0.00 / g
- 99%
- Cangzhou Kangrui Pharma Tech Co. Ltd.,