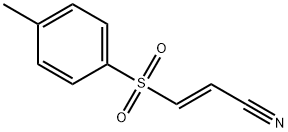
BAY 11-7082
- CAS No.
- 19542-67-7
- Chemical Name:
- BAY 11-7082
- Synonyms
- BAY E;CS-608;100824;BAY-11708;BAY 11-7821;BAY 11-7082;BAY 11-7082, >=98%;BAY11-7082/BAY117082;BAY11-7082,BAY 117821;BAY 11-7082 USP/EP/BP
- CBNumber:
- CB9733804
- Molecular Formula:
- C10H9NO2S
- Molecular Weight:
- 207.25
- MDL Number:
- MFCD00712162
- MOL File:
- 19542-67-7.mol
- MSDS File:
- SDS
| Melting point | 133-135℃ |
|---|---|
| Boiling point | 397.6±42.0 °C(Predicted) |
| Density | 1.237±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO: 25 mg/mL, soluble |
| form | solid |
| color | white |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| FDA UNII | 4Y5G2A4F6O |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319 | |||||||||
| Precautionary statements | P501-P270-P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313-P301+P312+P330 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UD1430000 | |||||||||
| HS Code | 2926907090 | |||||||||
| NFPA 704 |
|
BAY 11-7082 price More Price(42)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | B5556 | Bay 11-7082 ≥98% (HPLC), powder | 19542-67-7 | 10mg | $127 | 2024-03-01 | Buy |
| Sigma-Aldrich | 196870 | BAY 11-7082 | 19542-67-7 | 10mg | $113 | 2024-03-01 | Buy |
| TCI Chemical | T2846 | BAY 11-7082 >98.0%(HPLC)(N) | 19542-67-7 | 100mg | $49 | 2024-03-01 | Buy |
| TCI Chemical | T2846 | BAY 11-7082 >98.0%(HPLC)(N) | 19542-67-7 | 1g | $294 | 2024-03-01 | Buy |
| Cayman Chemical | 10010266 | BAY 11-7082 ≥98% | 19542-67-7 | 5mg | $49 | 2024-03-01 | Buy |
BAY 11-7082 Chemical Properties,Uses,Production
Description
BAY 11-7082 (19542-67-7)?inhibits cytokine-induced IκB-α phosphorylation via inhibition of IκB Kinase which results in inhibition of NFκB .1 Inhibits anchorage-independent growth of mammary epithelial cells induced with 4-hydroxyestradiol via inhibition of NFκB activation.2 Inhibits IFNα production and blocks nuclear translocation of IRF7 in plasmacytoid dendritic cells.3? Facilitates wound healing by inhibiting TNFα-induced MMP expression.4 A useful tool for probing the involvement of NFκB in physiological and pathophysiological processes.5? Cell permeable.
Uses
Bay 11-7082 has been used as:
- a nuclear factor-kappa B (NF-kB) inhibitor to verify the action of the NF-kB signaling pathway in the production of interleukin (IL)-8
- a nuclear factor-kappa B (NF-kB) inhibitor to study the role of NF-kB activation in Mycoplasma hyorhinis -induced epithelial-mesenchymal transition (EMT) and cell migration
- a nod-like receptor family pyrin domain containing 3 (NLRP3) selective inhibitor to examine its effects on liver inflammation in mice after hematopoietic stem cell transplantation
Definition
ChEBI: A nitrile that is acrylonitrile in which the hydrogen located beta,trans to the cyano group is replaced by a tosyl group. It is an inhibitor of cytokine-induced IkappaB-alpha phosphorylation i cells.
General Description
Potential anti-inflammatory agent that selectively and irreversibly inhibits the TNF-α-inducible phosphorylation of IκBα (IC50 = 10 μM), resulting in a decreased expression of NF-κB and of adhesion molecules. Does not affect constitutive IκBα autophosphorylation. Inhibits TNF-α-induced surface expression of the endothelial-leukocyte cell adhesion molecules E-selectin, VCAM-1, and ICAM-1. A 100 mM (10 mg/483 μl) solution of BAY 11-7082 (Cat. No. 196871) in DMSO is also available is also available.
Biological Activity
Irreversible inhibitor of TNF- α -stimulated I κ B α phosphorylation (IC 50 ~ 10 μ M); leads to decreased NF- κ B and subsequent decreased expression of adhesion molecules. Also reversibly activates MAP kinases and stimulates apoptosis.
Biochem/physiol Actions
Bay 11-7082 acts as a selective inhibitor for nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome pathway. In addition, to the inhibition of nuclear factor-kappa B (NF-kB), Bay 11-7082 also triggers apoptosis in anucleated erythrocytes, human T-cell leukemia virus type I (HTLV-I)-infected T-cell lines and primary adult T-cell leukemia cells.
Enzyme inhibitor
This protein kinase inhibitor (FW = 207.31 g/mol; CAS 19542-67-7; lmax = 251 nm), also named 3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile, targets NF-κB activation, selectively and irreversibly blocking TNF-α- induced phosphorylation of IκB-α without affecting phosphorylation of constitutive IκB-α. Mechanism of Inhibitory Action: NF-κB transcription factor regulates expression of inflammatory cytokines, various chemokines and immunoreceptors, as well as cell adhesion molecules. When stationed within the cytoplasm, NF-κB is kept inactive through its binding to the inhibitory factor IκB; however, certain stimuli result in IκB phosphorylation and ubiquitin-mediated degradation, freeing NF-κB for translocation to the nucleus. In endothelial cells, IκB-α phosphorylation and degradation occur within 15 min of TNFα treatment, allowing NF-κB to translocate to the nucleus to activate gene expression. Treatment of humnan vascular endothelial cells (HUVEC) with TNFα results in rapid loss of IκB-α from the cytoplasm. BAY11-7082 stabilizes IκB-α in a dose-dependent manner (IC50 ≈ 10 μM). There is a clear correlation between the concentration of drug that stabilizes IκB-α, the concentration that inhibits nuclear levels of NF-κB, and the concentration that inhibits adhesion molecule expression. More recent studies demonstrate that BAY 11- 7082 prevents ubiquitin conjugation to Ubc13 and UbcH7 by forming a covalent adduct with their cysteine residues via Michael addition at the C-3 atom of BAY 11-7082, followed by the release of 4-methylbenzenesulfinate. BAY 11-7082 stimulated Lys48-linked polyubiquitin chain formation in cells and protected Hypoxia-Inducible Factor-1α (HIF1α) from proteasomal degradation, suggesting it inhibits the proteasome. These results indicate that the anti-inflammatory effects of BAY 11-7082, its ability to induce B-cell lymphoma and leukemic T-cell death and to prevent the recruitment of proteins to sites of DNA damage are exerted via inhibition of components of the ubiquitin system– not by inhibiting NF-κB. BAY11-7082 also inhibits proliferation and promotes apoptosis in breast carcinoma MCF-7 cells by inhibiting phosphorylation of ATP citrate lyase.
storage
+4°C
References
1) Pierce et al. (1997) Novel inhibitor of cytokine-induced IkBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo; J. Biol. Chem., 272 21096 2) Park et al. (2009) 4-hydroxyestradiol induces anchorage-independent growth of human mammary epithelial cells via activation of IkappaB kinase: potential role of reactive oxygen species; Cancer Res., 69 2416 3) Miyamoto et al. (2010) Inhibitor of IkappaB kinase activity, BAY 11-7082, interferes with interferon regulatory factor 7 nuclear translocation and type I interferon production by plasmacytoid dendritic cells; Arthritis Res. Ther., 12 R87 4) Xu et al. (2019) Bay 11-7082 facilitates wound healing by antagonizing mechanical injury – and TNF-α-induced expression of MMPs in posterior cruciate ligament; Connect. Tissue Res. 60 311 5) Kim et al. (2018) TNF-α induces human neural progenitor cell survival after oxygen-glucose deprivation by activating the NFκB pathway; Exp. Mol. Med., 50 14
BAY 11-7082 Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29811 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22783 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10473 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| Nanjing Doge Biomedical Technology Co., Ltd | +86-25-58227606 +86-15305155328 | sales@dogechemical.com | China | 4128 | 58 |
View Lastest Price from BAY 11-7082 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | BAY 11-7082
19542-67-7
|
US $43.00-97.00 / mg | 99.92% | 10g | TargetMol Chemicals Inc. | ||
 |
2020-02-01 | BAY 11-7082
19542-67-7
|
US $3.00 / KG | 1KG | 98% | 100KG | Career Henan Chemical Co |
-

- BAY 11-7082
19542-67-7
- US $43.00-97.00 / mg
- 99.92%
- TargetMol Chemicals Inc.
-

- BAY 11-7082
19542-67-7
- US $3.00 / KG
- 98%
- Career Henan Chemical Co