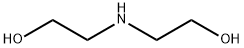
Triethanolamine
- CAS No.
- 102-71-6
- Chemical Name:
- Triethanolamine
- Synonyms
- TROLAMINE;Triethanolamin;Alkanolamine;N(CH2CH2OH)3;Nitrilotriethanol;Trola;Alkano;TRIETHANOLAMINE BUFFER;tris(Hydroxyethyl)amine;TEA AR
- CBNumber:
- CB9852620
- Molecular Formula:
- C6H15NO3
- Molecular Weight:
- 149.19
- MDL Number:
- MFCD00002855
- MOL File:
- 102-71-6.mol
- MSDS File:
- SDS
| Melting point | 17.9-21 °C (lit.) |
|---|---|
| Boiling point | 190-193 °C/5 mmHg (lit.) |
| Density | 1.124 g/mL at 25 °C (lit.) |
| vapor density | 5.14 (vs air) |
| vapor pressure | 0.01 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 365 °F |
| storage temp. | Store at RT. |
| solubility | H2O: 1 M, clear, colorless |
| form | Oily Liquid |
| Specific Gravity | 1.125 (20/20℃) |
| color | Clear colorless to slightly yellow |
| PH Range | 7.3 - 8.3 |
| Odor | Mild ammoniacal. |
| PH | 10.5-11.5 (25℃, 1M in H2O) |
| pka | 7.8(at 25℃) |
| explosive limit | 3.6-7.2%(V) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive & Hygroscopic |
| λmax | λ: 280 nm Amax: 0.1 |
| Merck | 14,9665 |
| BRN | 1699263 |
| Exposure limits | ACGIH: TWA 5 mg/m3 |
| Dielectric constant | 6.9(40℃) |
| InChIKey | GSEJCLTVZPLZKY-UHFFFAOYSA-N |
| LogP | -2.3 at 25℃ |
| Substances Added to Food (formerly EAFUS) | TRIETHANOLAMINE |
| FDA 21 CFR | 173.315; 175.105; 175.300; 176.170; 176.180; 176.200; 177.2600; 177.2800; 178.3910; 310.545; 352.70 |
| CAS DataBase Reference | 102-71-6(CAS DataBase Reference) |
| EWG's Food Scores | 3-5 |
| FDA UNII | 9O3K93S3TK |
| ATC code | D03AX12 |
| NIST Chemistry Reference | Triethanolamine(102-71-6) |
| IARC | 3 (Vol. 77) 2000 |
| EPA Substance Registry System | Triethanolamine (102-71-6) |
| Cosmetics Info | Triethanolamine |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38-36 | |||||||||
| Safety Statements | 26-39-36 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | KL9275000 | |||||||||
| F | 3-10-23 | |||||||||
| Autoignition Temperature | 600 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29221310 | |||||||||
| HS Code | 29321900 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rabbit > 2000 mg/kg | |||||||||
| NFPA 704 |
|
Triethanolamine price More Price(52)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T2545000 | Trolamine European Pharmacopoeia (EP) Reference Standard | 102-71-6 | 2EA | $152 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.22341 | Triethanolamine EMPLURA? | 102-71-6 | 25L | $636 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.08372 | Triethanolamine (Trolamine) | 102-71-6 | 1L | $289 | 2024-03-01 | Buy |
| Sigma-Aldrich | 33729 | Triethanolamine puriss. p.a., ≥99% (GC) | 102-71-6 | 1l | $138 | 2024-03-01 | Buy |
| Sigma-Aldrich | 50926 | Triethanolamine analytical reference material | 102-71-6 | 1ml | $85.6 | 2024-03-01 | Buy |
Triethanolamine Chemical Properties,Uses,Production
General description
Triethanolamine is a colorless oily liquid with the smell of ammonia. It is easy to absorb water and will turn into brown color when being exposed to the air and the light. At low temperature, it will become colorless or pale yellow cubic crystal. It is miscible with water, methanol and acetone. It is soluble in benzene, ether, slightly soluble in carbon tetrachloride, n-heptane. It is a kind of strong alkaline, combining with protons, can be used for condensation reaction.
Early strength agent
Triethanolamine is currently a commonly used early strength agent used in China's cement industry with the effect of early strength agent being accelerating hydration process of the cement in the presence of liquid phase in the concrete to improve the early strength. Although triethanolamine does not change the hydration product of cement, it can enhance the activity of the colloid generated through the hydration of cement, producing pressure to surrounding regions, blocking the capillary channel, exacerbating the effect of the adsorption, wetting and dispersion of particles and so on, promoting the reaction of the formation of hydrated calcium sulfoaluminate between the C3A and gypsum. This can improve the density of concrete, anti-permeability and antifreeze property, playing the role of early strength and enhancing the strength.
When used in combination with inorganic salts, it can play a catalytic role due to the hydration of cement itself and the reaction between inorganic salts and cement, so that the effect of early strength is particularly significant in the case.
Chemical properties
At room temperature, it appears as colorless transparent viscous liquid with hygroscopicity and ammonia smell. It is alkaline, being irritating. It has a melting point of 21.2 °C, the boiling point of 360 °C, a flash point of 193 ° C, the relative density (d420) 1.1242 and refractive index (nD20) of 1.4852. It is miscible with water, ethanol and acetone, slightly soluble in ether, benzene and carbon tetrachloride.
Uses
In analytical chemistry, triethanolamine can be used as the stationary phase for the gas liquid chromatography (the maximum temperature is 75 ℃ with the solvent being methanol and ethanol), used for the separation of pyridine and methyl substitutes. In the complexometric titration and other analysis, it can be used as a masking agent for interfering ions. For example, in a solution of pH = 10, when we apply EDTA for titration of magnesium, zinc, cadmium, calcium, nickel and other ions, the reagent can be used for masking titanium, aluminum, iron, tin and some other ions. In addition, it can also be dubbed with hydrochloric acid into a buffer solution of a certain pH value.
Triethanolamine is mainly used in the manufacture of surfactants, liquid detergents, cosmetics and so on. It is one of the components of cutting fluid and antifreeze fluid. During the nitrile rubber polymerization, it can be used as an activator, being the vulcanization activator of natural rubber and synthetic rubber. It can also be used as the emulsifiers of oil, wax and pesticides, the moisturizer and stabilizer of cosmetics, textile softeners as well as the anti-corrosion additives of lubricants. Triethanolamine is also capable of absorbing carbon dioxide and hydrogen sulfide and other gases. During the cleaning of the coke oven gas and other industrial gases, it can be used for removal of acid gases. It is also a commonly used masking agent in the EDTA titration assay.
Production method
Feed the ethylene oxide and ammonia water are into the reactor; conduct the condensation reaction under a reaction temperature of 30-40 °C and a reaction pressure of 70.9-304 kPa to generate a mixture solution of mono-, di-and triethanolamine; after undergoing dehydration and concentration at 90-120 °Cand then send to three vacuum distillation tower for vacuum distillation; capture different fractions according to different boiling points, you can get over 99% purity of the finished product of ethanolamine, diethanolamine and triethanolamine. During the course of the reaction, if increase the proportion of ethylene oxide, the generation ratio of di-and tri-ethanolamine will increase so we can get higher di-and tri-ethanolamine yield.
It is manufactured through the condensation reaction between ethylene oxide and ammonia under 30~40 °C and the pressure 71~304 kPa, in which the molar concentration of ethylene oxide and ammonia ratio is about 2.0. After the reaction, perform vacuum distillation through the distillation column, cut off the fractions of about 360 °C.
Description
Triethanolamine is a viscous, colourless/pale yellow liquid with a weak ammoniacal odour. Triethanolamine is incompatible with copper, copper alloys, galvanised iron, acids, and oxidisers. Reports indicate that in India itself, as many as six companies manufacture triethanolamine and it is manufactured by many different countries around the world. Global production and industrial application of triethanolamine is very extensive.

In industries, triethanolamine is used as a corrosion inhibitor in metal-cutting fluids; a curing agent for epoxy and rubber polymers; a copper–triethanolamine; in emulsifiers, thickeners, and wetting agents in the formulation of consumer products such as cosmetics, detergents, shampoos, and other personal products; and a neutraliser-dispersing agent in agricultural herbicide formulations. In brief, triethanolamine has wide applications as a corrosion inhibitor, a surface-active agent, and an intermediate in various products including metalworking fluids, oils, fuels, paints, inks, cement, cosmetic, and personal products and formulations of algicides and herbicides.
Chemical Properties
Triethanolamine is a pale yellow and viscous liquid. It is hygroscopic with an irritant and ammoniacal odor. There are multiple industrial and domestic applications for this compound, i.e., in the manufacture of toilet products, cosmetics formulations, solvents for waxes, resins, dyes, paraffi ns and polishes, herbicides, and lubricants for textile products. In the pharmaceutical industry, triethanolamine is used as a non-steroidal, antiinfl ammatory agent, an emulsifi er, and an alkylating agent.
Uses
The emulsifing agent triethanolamine can be contained in many products, such as metalwork cutting fluids and in color-film developers. Traces may exist in other ethanolamines such as mono- and diethanolamine.
Uses
Triethanolamine is used in fatty-acid soaps; in dry cleaning, cosmetics, shampoos, creams, waxes, cutting oils, household detergents, and emulsions; in wool scouring; textile antifume agent; water repellant; dispersion agent; corrosion inhibitor; softener; emulsifier; humectant; plasticizer; chelating agent; rubber accelerator; pharmaceutical alkalizing agent; catalyst for condensation etc.; in emulsions with mineral and vegetable oils.
Uses
Triethanolamine is used primarily as a surfactant, reducing the surface tension between two media. It is also used as a general emulsifier for preparations, such as ones involving drug penetration ass ays.
Definition
ChEBI: Triethanolamine is a tertiary amino compound that is ammonia in which each of the hydrogens is substituted by a 2-hydroxyethyl group. It has a role as a buffer and a surfactant. It is a tertiary amino compound, a triol and an amino alcohol. It is functionally related to a triethylamine. It is a conjugate base of a triethanolammonium.
Preparation
Triethanolamine is prepared commercially by the ammonolysis of ethylene oxide. The reaction yields a mixture of monoethanolamine, diethanolamine, and triethanolamine, which are separated to obtain the pure products.
Production Methods
Triethanolamine is produced with ethanolamine and diethanolamine by ammonolysis of ethylene oxide and the triethanolamine is then separated by distillation (Mullins 1978). In 1984, 139.6 million pounds of triethanolamine were produced in the United States (USTIC 1985).
brand name
Mobisyl [as salicylate] (Ascher);Sabrilex.
Composition
The dried leaves contain protein, 25.7%; fat, 6.5%; carbohydrate, 40.8%; ash, 5%; caffeine, 3.3% and tannin, 13%. The most common catechins are gallic esters (epicatechin, epicatechin gallate and epigallocatechin gallate). All are found in green tea and are claimed to be responsible for the chemopreventive benefits of the beverage.
World Health Organization (WHO)
Trolamine is widely used as an emulsifier in combination with fatty acids in pharmaceutical and cosmetic products. The World Health Organization is not aware of restrictive action having been taken elsewhere.
General Description
Oily liquid with a mild ammonia odor. Denser than water. Freezing point is 71°F.
Air & Water Reactions
Water soluble.
Reactivity Profile
Triethanolamine is an aminoalcohol. Neutralize acids to form salts plus water in exothermic reactions. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides. Reacts violently with strong oxidants. [Handling Chemicals Safely 1980. p. 928].
Health Hazard
Exposures to triethanolamine, in contrast with other chemical compounds, is known to cause low toxicity to animals and the acute oral LD50 to rats and guinea pigs ranges from 8000 to 9000 mg/kg. Triethanolamine was found to be a moderate eye irritant. A 5%–10% solution of triethanolamine did not induce skin irritation or skin sensitization. Studies of Inoue et al. and many other workers have indicated the absence of the mutagenic potential of triethanolamine as evidenced by both in vivo and in vitro studies (Salmonella typhimurium tests, Chinese hamster ovary cells, and rat liver chromosome analysis). Further, extensive studies have demonstrated the absence of potential carcinogenicity of triethanolamine in rats and mice, suggesting a low or lack of acute or chronic toxicity of the chemical to mammals.
Fire Hazard
Special Hazards of Combustion Products: Poisonous gases, such as NOx, may be produced
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Dilute with water; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Pharmaceutical Applications
Triethanolamine is widely used in topical pharmaceutical formulations,
primarily in the formation of emulsions.
When mixed in equimolar proportions with a fatty acid, such as
stearic acid or oleic acid, triethanolamine forms an anionic soap
with a pH of about 8, which may be used as an emulsifying agent to
produce fine-grained, stable oil-in-water emulsions. Concentrations
that are typically used for emulsification are 2–4% v/v of
triethanolamine and 2–5 times that of fatty acids. In the case of
mineral oils, 5% v/v of triethanolamine will be needed, with an
appropriate increase in the amount of fatty acid used. Preparations
that contain triethanolamine soaps tend to darken on storage.
However, discoloration may be reduced by avoiding exposure to
light and contact with metals and metal ions.
Triethanolamine is also used in salt formation for injectable
solutions and in topical analgesic preparations. It is also used in sun
screen preparations.
Triethanolamine is used as an intermediate in the manufacturing
of surfactants, textile specialties, waxes, polishes, herbicides,
petroleum demulsifiers, toilet goods, cement additives, and cutting
oils. Triethanolamine is also claimed to be used for the production
of lubricants for the rubber gloves and textile industries. Other
general uses are as buffers, solvents, and polymer plasticizers, and as
a humectant.
Industrial uses
Triethanolamine undergoes reactions characteristic of tertiary amines and of
alcohols. Two industrially important reactions of the ethanolamines involve
reaction with carbon dioxide or hydrogen sulfide to yield water soluble salts, and
reaction with long chain fatty acids to form neutral ethanolamine soaps (Mullins
1978). Substituted ethanolamine compounds, such as soaps, are used extensively
as emulsifiers, thickeners, wetting agents, and detergents in cosmetic formulations
(including skin cleaners, creams, and lotions) (Beyer et al 1983).
The largest uses for triethanolamine are in the production of fatty acid soaps and
detergents and in cosmetic formulations. In cosmetics, triethanolamine is an
important raw material and is used in combination with fatty acids as emulsifiers
for creams, lotions, skin cleaners, and shampoos. Triethanolamine is also used in
cement and concrete to reduce particle agglomeration within the grinding mill; as
an antistatic agent in the textile industry; in the metal industry for metal plating
and in alkaline derusting formulations; in the rubber industry as a vulcanization
accelerator; and in the manufacture of herbicides and pesticides. Triethanolamine
may also be used as a surface active agent in cutting fluids; as an absorption agent
for acidic gases in air pollution control; as a component of coating on fruits and
vegetables; as a solvent for casein, shellac, and dyes; and as a penetrating agent for
organic liquids in wood and paper (Bayer et al 1983; Mullins 1978; Windholz
1983). Triethanolamine is permitted in articles intended for use in the production,
processing, or packaging of food (CFR 1981).
Contact allergens
This emulsifying agent can be contained in many products such as cosmetics, topical medicines, metalworking cut- ting fluids, and color film developers. Traces may exist in other ethanolamines such as monoand diethanolamine. Contact allergy seems to be rarer than previously thought.
Safety Profile
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. Liver and kidney damage have been demonstrated in animals from chronic exposure. A human and experimental skin irritant. An eye irritant. Questionable carcinogen with experimental carcinogenic data. Combustible liquid when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx and CN-.
Safety
Triethanolamine is used primarily as an emulsifying agent in a
variety of topical pharmaceutical preparations. Although generally
regarded as a nontoxic material, triethanolamine may cause
hypersensitivity or be irritant to the skin when present in formulated
products. The lethal human oral dose of triethanolamine is
estimated to be 5–15 g/kg body-weight.
Following concern about the possible production of nitrosamines
in the stomach, the Swiss authorities have restricted the use of
triethanolamine to preparations intended for external use.
LD50 (guinea pig, oral): 5.3 g/kg
LD50 (mouse, IP): 1.45 g/kg
LD50 (mouse, oral): 7.4 g/kg
LD50 (rat, oral): 8 g/kg
Potential Exposure
Monoethanolamine is widely used in industry for scrubbing acid gases and in production of detergents and alkanolamide surfactants; to remove carbon dioxide and hydrogen from natural gas, to remove hydrogen sulfide and carbonyl sulfide; as an alkaline conditioning agent; as an intermediate for soaps, detergents, dyes, and textile agents. Diethanolamine is an absorbent for gases; a solubilizer for 2,4- dichlorophenoxyacetic acid (2,4-D); and a softener and emulsifier intermediate for detergents. It also finds use in the dye and textile industry. Triethanolamine is used as plasticizers, neutralizer for alkaline dispersions; lubricant additive; corrosion inhibitor; and in the manufacture of soaps, detergents, shampoos, shaving preparations; face and hand creams; cements, cutting oils, insecticides, surface active agents; waxes, polishes, and herbicides.
Carcinogenicity
Results of carcinogenicity studies have been controversial. Hoshino and Tanooka reported that triethanolamine in the diet of mice at levels of 0.03% or 0.3% caused a significant increase in the occurrence of tumors, both benign and malignant. Females showed a 32% increase, mostly of thymic lymphomas. The increase of all other tumors, in both sexes, was 8.2%. They also found that triethanolamine reacted with sodium nitrite to produce N-nitrosodiethanolamine and that the product caused mutagenesis in bacteria. Maekawa et al. reported that no carcinogenic activity was found when given orally to rats in drinking water at concentrations of 1% and 2% for 2 years. However, the dosage to females was halved after week 69 of treatment owing to nephrotoxicity. Histological examination of renal damage in treated animals revealed acceleration of chronic nephropathy, mineralization of the renal papilla, nodular hyperplasia of the pelvic mucosa, and pyelonephritis with or without papillary necrosis. Nephrotoxicity seemed to affect life span adversely, especially in females. Tumor incidence and histology were the same in the treated group as in controls.
storage
Triethanolamine may turn brown on exposure to air and light.
The 85% grade of triethanolamine tends to stratify below 15℃;
homegeneity can be restored by warming and mixing before use.
Triethanolamine should be stored in an airtight container
protected from light, in a cool, dry place.
See Monoethanolamine for further information.
Shipping
UN2491 Ethanol amine or Ethanolamine solutions, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Shake the amine gently with Linde type 4A molecular sieves for 24hours, filter and fractionate it under a vacuum, and preferably in the presence of N2. Store it in dark stoppered bottles under N2 as it is hygroscopic, and turns brown in air and light. It has a strong ammoniacal odour (like diethanolamine). It is miscible with H2O, MeOH and Me2CO, and its solubilities at 25o in n-heptane, Et2O and *C6H6 are 0.4%, 1.6% and 4.2%, respectively. [See diethanolamine above, Beilstein 4 IV 1524.]
Incompatibilities
Triethanolamine is a tertiary amine that contains hydroxy groups; it
is capable of undergoing reactions typical of tertiary amines and alcohols. Triethanolamine will react with mineral acids to form
crystalline salts and esters. With the higher fatty acids, triethanolamine
forms salts that are soluble in water and have characteristics
of soaps. Triethanolamine will also react with copper to form
complex salts. Discoloration and precipitation can take place in the
presence of heavy metal salts.
Triethanolamine can react with reagents such as thionyl chloride
to replace the hydroxy groups with halogens. The products of these
reactions are very toxic, resembling other nitrogen mustards.
Waste Disposal
Controlled incineration; incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions
Regulatory Status
Included in the FDA Inactive Ingredients Database (rectal, topical, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Triethanolamine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 | admin@hbsaisier.cn | China | 1015 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 +86-18633929156 | admin@hblongbang.com | China | 941 | 58 |
| Qingdao RENAS Polymer Material Co., Ltd. | +86-0532-86867058 +86-18562606086 | sales@qdrenas.com | China | 476 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 | L18532138899@163.com | China | 939 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615350851019 | admin@86-ss.com | China | 1001 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
Related articles
- What is Triethanolamine used for in pharmaceuticals and skin care products?
- Triethanolamine (TEA) is used in the pharmaceutical field as an excipient or solubiliser in the formulation of topical and ora....
- Sep 13,2024
- Preparation and Applications of Triethanolamine
- Triethanolamine is a colorless to yellow liquid. It is completely soluble in water and has an ammonia-like smell. Building blo....
- Jul 13,2022
- What is Triethanolamine?
- Triethanolamine is used in fatty-acid soaps; in dry cleaning, cosmetics, shampoos, creams, waxes, cutting oils, household dete....
- Jul 20,2020
View Lastest Price from Triethanolamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-29 | Triethanolamine
102-71-6
|
US $6.00 / kg | 1kg | More than 99% | 2000KG/Month | Hebei Longbang Technology Co., Ltd | |
 |
2024-11-29 | Triethanolamine
102-71-6
|
US $100.00-75.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-29 | Triethanolamine
102-71-6
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd |
-

- Triethanolamine
102-71-6
- US $6.00 / kg
- More than 99%
- Hebei Longbang Technology Co., Ltd
-

- Triethanolamine
102-71-6
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Triethanolamine
102-71-6
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd