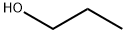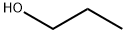1-Propanol
- CAS No.
- 71-23-8
- Chemical Name:
- 1-Propanol
- Synonyms
- PROPANOL;N-PROPANOL;PROPAN-1-OL;Propyl alcohol;N-PROPYL ALCOHOL;Propanol-1;1-Propyl alcohol;PROPANE-1-OL;n-Propan-1-ol;n-C3H7OH
- CBNumber:
- CB9854149
- Molecular Formula:
- C3H8O
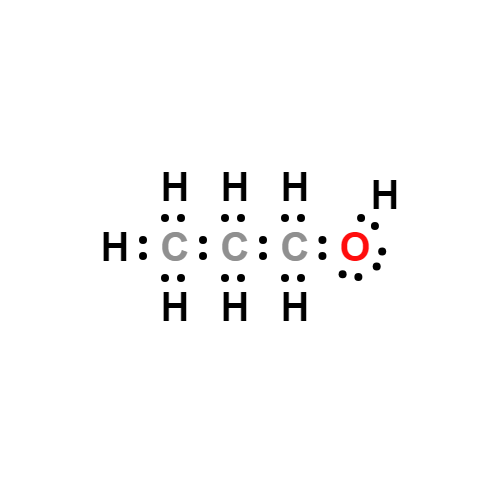
Lewis structure
- Molecular Weight:
- 60.1
- MDL Number:
- MFCD00002941
- MOL File:
- 71-23-8.mol
- MSDS File:
- SDS
| Melting point | -127 °C(lit.) |
|---|---|
| Boiling point | 97 °C(lit.) |
| Density | 0.804 g/mL at 25 °C(lit.) |
| vapor density | 2.1 (vs air) |
| vapor pressure | 10 mm Hg ( 147 °C) |
| FEMA | 2928 | PROPYL ALCOHOL |
| refractive index |
n |
| Flash point | 59 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: passes test |
| form | Liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| color | <10(APHA) |
| Odor | Resembles that of ethyl alcohol. |
| Relative polarity | 0.617 |
| PH | 7 (200g/l, H2O, 20℃) |
| explosive limit | 2.1-19.2%(V) |
| Odor Threshold | 0.094ppm |
| Odor Type | alcoholic |
| Evaporation Rate | 1.3 |
| Viscosity | 2.27 mPas at 20°C |
| Water Solubility | soluble |
| λmax |
λ: 220 nm Amax: ≤0.40 λ: 240 nm Amax: ≤0.071 λ: 275 nm Amax: ≤0.0044 |
| Merck | 14,7842 |
| JECFA Number | 82 |
| BRN | 1098242 |
| Henry's Law Constant | 6.75 (static headspace-GC, Merk and Riederer, 1997) |
| Exposure limits | TLV-TWA (200 ppm); (500 mg/m3); STEL 250 ppm (625 mg/m3); IDLH 4000 ppm. |
| Dielectric constant | 20.1(25℃) |
| Stability | Stable. May form peroxides in contact with air. Incompatible with alkali metals, alkaline earths, aluminium, oxidizing agents, nitro compounds. Highly flammable. Vapour/air mixtures explosive. |
| LogP | 0.33 |
| Substances Added to Food (formerly EAFUS) | PROPYL ALCOHOL |
| FDA 21 CFR | 177.1200 |
| CAS DataBase Reference | 71-23-8(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 96F264O9SV |
| ATC code | D08AX03,D08AX53 |
| NIST Chemistry Reference | 1-Propanol(71-23-8) |
| EPA Substance Registry System | 1-Propanol (71-23-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H318-H336 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P280-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xi | |||||||||
| Risk Statements | 11-41-67 | |||||||||
| Safety Statements | 7-16-24-26-39 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 200 ppm (500 mg/m3), STEL: 250 ppm (625 mg/m3) [skin] | |||||||||
| RIDADR | UN 1274 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | UH8225000 | |||||||||
| F | 10-23 | |||||||||
| Autoignition Temperature | 700 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29051200 | |||||||||
| Toxicity | LD50 orally in rats: 1.87 g/kg (Smyth) | |||||||||
| IDLA | 800 ppm | |||||||||
| NFPA 704 |
|
1-Propanol price More Price(89)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W292826 | 1-Propanol natural, ≥98%, FG | 71-23-8 | 1kg | $198 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292826 | 1-Propanol natural, ≥98%, FG | 71-23-8 | 4kg | $444 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292818 | 1-Propanol ≥99%, FG | 71-23-8 | 1kg | $76.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292818 | 1-Propanol ≥99%, FG | 71-23-8 | 4kg | $118 | 2024-03-01 | Buy |
| Sigma-Aldrich | W292818 | 1-Propanol ≥99%, FG | 71-23-8 | 20kg | $380 | 2024-03-01 | Buy |
1-Propanol Chemical Properties,Uses,Production
Description
1-propanol is the compound with the hydrogen atom in the propane molecules being replaced by hydroxyl group. Because the hydroxyl group can substitute the hydrogen atoms contained in the carbons in the two terminals of carbon chain or middle carbon, thus generating two isomers, n-propyl alcohol and isopropyl alcohol.
The chemical property of the 1-propanol is similar to that of ethanol. It is the byproduct during the process of the methanol synthesis from carbon monoxide and hydrogen. At room temperature and normal pressure, it appears as colorless transparent liquid with fragrance odor. In industry, it is prepared through the reaction between ethylene, carbon monoxide and hydrogen under high pressure and cobalt catalysis; alternatively through the hydration of propylene under the action of sulfate or through the catalytic hydrogenation of acetone. It is commonly used as a solvent with irritating effect on the eyes and mucosa. Inhalation of propyl alcohol steam can lead to dizziness, headache and vomiting, etc.
Chemical Properties
1-Propanol is a clear, colorless liquid with an alcoholic odor and a characteristic ripe, fruity flavor. It is soluble in water and miscible with organic solvents (Propanols). It has better dissolution properties than ethanol for fats and oils, and dissolves polar resins in the same way as ethanol. Cellulose nitrate and poly(vinyl acetate) are, however, almost insoluble. For economic reasons propanol is of only limited use as a solvent, and is a starting material for esters.
Physical properties
Colorless liquid with a mild, alcohol-like odor. Experimentally determined detection and recognition odor threshold concentrations were <75 μg/m3 (<31 ppbv) and 200 μg/m3 (81 ppbv), respectively (Hellman and Small, 1974). An odor threshold concentration of 100 ppbv was reported by Nagata and Takeuchi (1990).
Occurrence
Reported found in the natural aromas of apple, cognac and rum; also formed during alcoholic fermentation. Also reported found in apple, apricot, banana, sweet cherry, papaya, pineapple, orange juice, lingonberry, cranberry, grapes, peas, pineapple, raspberry, strawberry, onion, leek, tomato, ginger, vinegar, many cheeses, butter, fatty fish, fish oil, cooked beef, mutton and pork, beer, several types of bread, pear brandy, Scotch blended whiskey, malt whiskey, cognac, armagnac, weinbrand rum, bourbon whiskey, Irish whiskey, rum, grape wines, cider, sherry, cocoa, tea, roasted filberts and peanuts, honey, soybean, oats, passion fruit, plum, beans, mushroom, apple and plum brandy, gin, rice, rice bran, quince, prickly pear, jackfruit, sake, buckwheat, loquat, wild rice, anise brandy, endive, truffle, arrack, clam, cape gooseberry and Chinese quince.
Uses
A colorless liquid made by oxidation of aliphatic hydrocarbons that is used as a solvent and chemical intermediate.
Uses
1-Propanol is used in making n-propyl acetate; and as a solvent for waxes, resins, vegetable oils, and flexographic printing ink. It is produced from the fermentation and spoilage of vegetable matter.
Application
The propanols are used mainly as solvents for coatings; in antifreeze compositions and household and personal products; and as chemical intermediates for the production of esters, amines, and other organic derivatives. As a solvent, 1-propanol is used principally in printing inks, paint, cosmetics, pesticides, cellulose esters and insecticides.
1-Propanol is used commercially to produce glycol ethers. These are characterized by dual functionality, which imparts high solvency, chemical stability, and water compatibility.
Preparation
1-Propanol is produced commercially by the oxo process by reacting ethylene with carbon monoxide and hydrogen in the presence of a catalyst to give propionaldehyde, which is then hydrogenated. 1-Propanol [71-23-8] is the major product of catalytic reduction of propanal (→Propanols). Reduction is carried out most economically by a continuous vapor-phase process over a heterogeneous catalyst of supported reduced nickel, copper, and/or zinc and manganese metals.
Definition
ChEBI: 1-Propanol is the parent member of the class of propan-1-ols that is propane in which a hydrogen of one of the methyl groups is replaced by a hydroxy group. It has a role as a protic solvent and a metabolite. It is a short-chain primary fatty alcohol and a member of propan-1-ols.
Aroma threshold values
Detection: 5.7 to 40 ppm; recognition: 600 to 6300 ppm
General Description
N-propanol appears as a clear colorless liquid with a sharp musty odor like rubbing alcohol. Flash point 53-77 °F. Autoignites at 700 °F. Vapors are heavier than air and mildly irritate the eyes, nose, and throat. Density approximately 6.5 lb / gal. Used in making cosmetics, skin and hair preparations, pharmaceuticals, perfumes, lacquer formulations, dye solutions, antifreezes, rubbing alcohols, soaps, window cleaners, acetone and other chemicals and products.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
1-Propanol reacts with alkali metal, nitrides and strong reducing agents to give flammable and/or toxic gases. Reacts with oxoacids and carboxylic acids to form esters plus water. Converted by oxidizing agents to propanal or propionic acid. May initiate the polymerization of isocyanates and epoxides. Incompatible with strong oxidizing agents .
Hazard
Flammable, dangerous fire risk. Explosive limits in air 2–13%. Toxic by skin absorption. Eye and upper respiratory tract irritant. Questionable carcinogen.
Health Hazard
Target organs: skin, eyes, gastrointestinal tracts, and respiratory system. Toxic routes: ingestion, inhalation, and skin contact.
LD50 value, oral (rats): 5400 mg/kg (NIOSH1986)
LD50 value, skin (rabbits): 6700 mg/kg(NIOSH 1986)
Ingestion causes headache, drowsiness,abdominal cramps, gastrointestinal pain,ataxia, nausea, and diarrhea. Eye contactproduces irritation. It may cause dermatitison repeated skin contact. Although thetoxicity of 1-propanol is low, at a highconcentration it may produce a narcoticeffect, as well as irritation of the eyes, nose,and throat..
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Biochem/physiol Actions
Taste at 25-250 ppm
Safety Profile
Poison by subcutaneous route. Moderately toxic by inhalation, ingestion, intraperitoneal, and intravenous routes. A skin and severe eye irritant. Questionable carcinogen with experimental carcinogenic data. Mutation data reported. A flammable liquid and dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosive in the form of vapor when exposed to heat or flame. Ignites on contact with potassium-tert- butoxide. Dangerous upon exposure to heat or flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
n-Propyl alcohol is used as as solvent in lacquers, dopes; to make cosmetics; dental lotions; clea- ners, polishes, and pharmaceuticals; as a surgical antiseptic. It is a solvent for vegetable oils, natural gums and resins; rosin, shellac, certain synthetic resins; ethylcellulose, and butyral; as a degreasing agent; as a chemical intermediate.
Carcinogenicity
Eighteen Wistar rats were dosed by oral gavage with 0.3 mL/kg twice weekly. The average survival time was 570 days. In addition to severe liver injury and hyperplasia of the hematopoietic parenchyma, 5 malignant tumors (2 myeloid leukemias, 2 liver sarcomas, and 1 liver cell carcinoma) and 10 benign tumors were observed. Three benign but no malignant tumors were found in the controls given saline.
Environmental Fate
Biological. In activated sludge inoculum, following a 20-d adaptation period, 98.8% COD
removal was achieved. The average rate of biodegradation was 71.0 mg COD/g?h (Pitter, 1976).
Using the BOD technique to measure biodegradation, the mean 5-d BOD value (mM BOD/mM 1-
propanol) and ThOD were 2.70 and 60.0%, respectively (Vaishnav et al., 1987).
Photolytic. Reported rate constants for the reaction of 1-propanol and OH radicals in the atmosphere: 2.3 x 10-12 cm3/molecule?sec at 300 K (Hendry and Kenley, 1979); 2.3 x 10-9
L/molecule?sec (second-order) at 292 K (Campbell et al., 1976), 5.33 x 10-12 cm3/molecule?sec at
296 K (Overend and Paraskevopoulos, 1978). Based on an atmospheric OH concentration of 1.0 x
106 molecule/cm3, the reported half-life of 1-propanol is 1.5 d (Grosjean, 1997).
Chemical/Physical. At an influent concentration of 1,000 mg/L, treatment with GAC resulted in
an effluent concentration of 811 mg/L. The adsorbability of the carbon used was 38 mg/g carbon
(Guisti et al., 1974).
Shipping
UN1274, n-Propanol, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
The main impurities in n-propyl alcohol are usually water and 2-propen-1-ol, reflecting the commercial production by hydration of propene. Water can be removed by azeotropic distillation either directly (azeotrope contains 28% water) or by using a ternary system, e.g. by also adding *benzene. Alternatively, for removal of gross amounts of water, reflux over CaO for several hours is desirable, followed by distillation and a further drying. To obtain more nearly anhydrous alcohol, suitable drying agents are firstly NaOH, CaSO4 or K2CO3, then CaH2, aluminium amalgam, magnesium activated with iodine, or a small amount of sodium. Alternatively, the alcohol can be refluxed with n-propylsuccinate or phthalate in a method similar to the one described under EtOH. Allyl alcohol is removed by adding bromine (15mL/L) and then fractionally distilling from a small amount of K2CO3. Propionaldehyde, also formed in the bromination, is removed as the 2,4-dinitrophenylhydrazone. n-Propyl alcohol can be dried down to 20ppm of water by passage through a column of pre-dried molecular sieves (type 3 or 4A, heated for 3hours at 300o) in a current of nitrogen. Distillation from sulfanilic or tartaric acids removes impurities. Albrecht [J Am Chem Soc 82 3813 1960] obtained spectroscopically pure material by heating with charcoal to 50-60o, filtering and adding 2,4-dinitrophenylhydrazine and a few drops of conc H2SO4. After standing for several hours, the mixture is cooled to 0o, filtered and distilled in a vacuum. Gold and Satchell [J Chem Soc 1938 1963] heated n-propyl alcohol with 3-nitrophthalic anhydride at 76-110o for 15hours, then recrystallised the resulting ester from H2O, *benzene/pet ether (b 100-120o)(3:1), and *benzene. The ester was hydrolysed under reflux with aqueous 7.5M NaOH for 45minutes under nitrogen, followed by distillation (also under nitrogen). The fraction with b 87-92o is dried with K2CO3 and stirred under reduced pressure in the dark over 2,4-dinitrophenylhydrazine, then freshly distilled. Also purify it by adding 2g NaBH4 to 1.5L of alcohol, gently flushing with argon and refluxing for 1day at 50o. Then 2g of freshly cut sodium (washed with propanol) is added and refluxed for one day, and finally distilled, taking the middle fraction [Jou & Freeman J Phys Chem 81 909 1977]. [Beilstein 1 IV 1413.]
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, perox- ides, permanganates, perchlorates, chlorine, bromine, fluo- rine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. n-Propanol reacts with alkali metal, nitrides and strong reducing agents to give flammable and/ or toxic gases. Reacts with oxoacids and carboxylic acids to form esters plus water. Converted by oxidizing agents to propanal or propionic acid. May initiate the polymerization of isocyanates and epoxides
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
1-Propanol Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2737 | 58 |
| Shandong Quark Chemical Co., Ltd. | +8613325218432 | sales@quarkchem.com | China | 57 | 58 |
| PT CHEM GROUP LIMITED | peter68@ptchemgroup.com | China | 35425 | 58 | |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 985 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Qingdao RENAS Polymer Material Co., Ltd. | +86-0532-86867058 +86-18562606086 | sales@qdrenas.com | China | 476 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
Related articles
- The Polarity of 1-Propanol and Its Effect on Organic Systems
- 1-Propanol is a good solvent and can be used directly or through the synthesis of propyl acetate in coatings, printing inks, d....
- Dec 28,2023
View Lastest Price from 1-Propanol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-21 | 1-Propanol
71-23-8
|
US $10.00 / KG | 1KG | 99% | 100 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-11-07 | 1-Propanol
71-23-8
|
US $50.00 / kg | 1kg | 99% | 200000000 | Hebei Jingbo New Material Technology Co., Ltd | |
 |
2024-10-29 | 1-Propanol
71-23-8
|
US $0.00 / kg | 20kg | 99.0% | 20 tons | Qingdao RENAS Polymer Material Co., Ltd. |
-

- 1-Propanol
71-23-8
- US $10.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- 1-Propanol
71-23-8
- US $50.00 / kg
- 99%
- Hebei Jingbo New Material Technology Co., Ltd
-

- 1-Propanol
71-23-8
- US $0.00 / kg
- 99.0%
- Qingdao RENAS Polymer Material Co., Ltd.
71-23-8(1-Propanol)Related Search:
1of4