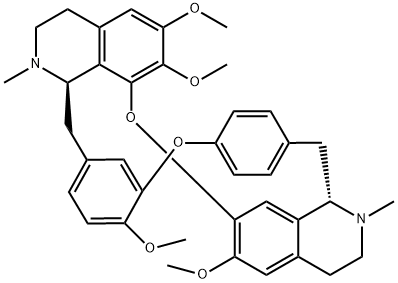
D-Tetrandrine
- CAS No.
- 518-34-3
- Chemical Name:
- D-Tetrandrine
- Synonyms
- TETRANDRINE;hanfangchin A;Conba;Jinake;Trandrine;fanchinine;TETRANDRIN;Tetradrine;sinomeninea;Tetrantrine
- CBNumber:
- CB9855050
- Molecular Formula:
- C38H42N2O6
- Molecular Weight:
- 622.75
- MDL Number:
- MFCD00082551
- MOL File:
- 518-34-3.mol
- MSDS File:
- SDS
| Melting point | 219-222 °C(lit.) |
|---|---|
| Boiling point | 662.81°C (rough estimate) |
| alpha | 285 º (c=1, CHCl3) |
| Density | 1.1528 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated) |
| form | solid |
| pka | 7.70±0.20(Predicted) |
| color | Off-White |
| λmax | 283nm(EtOH)(lit.) |
| Merck | 14,9231 |
| LogP | 3.550 (est) |
| EWG's Food Scores | 1 |
| FDA UNII | 29EX23D5AJ |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300 | |||||||||
| Precautionary statements | P264-P270-P301+P310-P405-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 22-24/25-36-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | XE9350000 | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
D-Tetrandrine price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | Y0001165 | Tetrandrine European Pharmacopoeia (EP) Reference Standard | 518-34-3 | y0001165 | $150 | 2024-03-01 | Buy |
| Sigma-Aldrich | T2695 | Tetrandrine analytical standard, for drug analysis | 518-34-3 | 1g | $253 | 2024-03-01 | Buy |
| TCI Chemical | T3321 | Tetrandrine >98.0%(HPLC)(T) | 518-34-3 | 200mg | $60 | 2024-03-01 | Buy |
| TCI Chemical | T3321 | Tetrandrine >98.0%(HPLC)(T) | 518-34-3 | 1g | $199 | 2024-03-01 | Buy |
| Cayman Chemical | 19874 | Tetrandrine ≥98% | 518-34-3 | 250mg | $71 | 2024-03-01 | Buy |
D-Tetrandrine Chemical Properties,Uses,Production
Description
Recently it has been discovered that pronounced drug-dependence and related toxic effects occur in both dogs and rhesus monkeys with this alkaloid on intravenous injection with a dose of 10-150 mg/kg. When rapidly injected, the acute hypotensive effect is very marked and fatal at once. Following drug administration at toxic levels it is found that severe local tissue reaction heptaotoxicity and lymphoid necrosis occurs. At the highest dosage level there is a very definite nephrotoxicity in monkeys and some indications of this in dogs. The evidence available suggests that monkeys are less sensitive to hepatotoxicity with this drug than dogs.
Description
Tetrandrine is a bis-benzylisoquinoline alkaloid that has been found in R. stephania roots and has diverse biological activities. It induces autophagy in HeLa, MCF-7, and human foreskin fibroblast (HFF) cells when used at a concentration of 5 μM, an effect that can be reversed by the autophagy inhibitor 3-methyladenine . Tetrandrine inhibits PAF-, thrombin-, collagen-, ADP-, or epinephrine-induced aggregation of isolated human platelets. Priming of mesenchymal stem cells (MSCs) with tetrandrine (5 and 10 μM) reduces TNF-α secretion by RAW 264.7 cells in co-culture. Ear skin transplantation of tetrandrine-primed MSCs decreases ear levels of TNF-α in a mouse model of ear skin inflammation. Tetrandrine (1 mg/kg) increases soleus muscle levels of glucose transporter 4 (Glut4) and decreases plasma glucose levels in a rat model of diabetes induced by streptozotocin .
Chemical Properties
White powder
Physical properties
Appearance: Needle-like crystals (ether). Solubility: Hardly soluble in water and petroleum ether; soluble in ether and some organic solvents. Melting point: 219– 222?°C. Specific optical rotation: 285° (c?=?1, CHCl3); sensitive to light.
History
Recent studies have shown that tetrandrine has a variety of biological effects and
very good applicational prospects in the treatment of fibrosis and portal vein and
pulmonary hypertension, the regulation of immunologic function, as well as the
prevention and treatment of tumor.
As early as 1988, tetrandrine has been found having the effect on blocking the
Ca2 + channel and was quickly applied into the pharmacological research in the field
of cardiovascular and inflammatory diseases. Results from a large number of
studies have shown that tetrandrine has good effects on antihypertension, arrhythmia, myocardial ischemia, inflammation, and so on. As a traditional Chinese
calcium antagonist, tetrandrine has a broad prospects in clinical applications of cardiovascular and inflammatory diseases.In the early 1990s, the application of tetrandrine was extended. During that time,
researchers conducted many studies about its protective effects on liver, lung, and
mitochondria, which opened a new field for the treatment of liver disease. In
2002, it was found that tetrandrine can inhibit the synthesis of DNA and RNA in
tumor cells, which provided a new method for the treatment of cancer.
At present, the prevention and treatment effects of hypertension, fibrosis, digestive diseases, tumors, rheumatoid arthritis, and other autoimmune diseases of tetrandrine have been confirmed, as well as the function of reducing portal hypertension
and pulmonary hypertension, while its other pharmacological effects are to be
explored in further study.
Uses
Tetrandrine is a lipopolysaccharide-induced microglial suppressor, effectively reducing the production of bacterial inflammatory mediators. Anti-inflammatory, anti-nociceptive. Tetrandrine is used in China to treat high blood pressure. This drug blocked the TPC2 calcium channel required for the Ebola infection process (Robert A. Davey et al., Science 2015, DOI: 10.1126/science.1258758).
Uses
analgesic, antineoplastic, antihypertensive, lymphotoxin
Indications
This product is included in national standards for chemical drugs (Volume 14), British Pharmacopoeia (2017), and European Pharmacopoeia (9.0th ed.). Tetrandrine is used for the treatment of mild to moderate hypertension and hypertensive crisis, rheumatism, silicosis, etc.
Definition
ChEBI: (+)-Tetrandrine is a member of isoquinolines and a bisbenzylisoquinoline alkaloid.
Pharmacology
Tetrandrine has analgesic, anti-inflammatory, and anti-allergic effects and has a wide range of usage on the cardiovascular system owing to its antihypertensive, anti-myocardial ischemia/reperfusion injury and antiarrhythmic effects. It can inhibit the platelet aggregation induced by ADP, collagen, and arachidonic acid in?vitro and can also restrain the platelet adhesion and thrombosis (in rabbits). Tetrandrine also has anticancer effects. Studies have shown that tetrandrine has a strong inhibitory effect on the DNA and RNA synthesis in L7712 and S180 (cancer cells), which can significantly suppress the growth of Wacker sarcoma W256. Besides that, tetrandrine has the ability to relax the striated muscle, and its methyl iodide or methyl bromide derivatives can also affect the muscles. Notably, tetrandrine can prevent silicosis and has a preferable outcome on the clinical treatment of such disease. In addition, tetrandrine also owns antipyretic, diuretic, and antiallergic shock effects.
Clinical Use
Tetrandrine is used for the treatment of hypertension, angina, termination of paroxysmal supraventricular tachycardia, pulmonary fibrosis, and other diseases in clinical application, and it also has strong antitumor effects. Tetrandrine was also approved for lowering blood glucose and free radical damage; its treatment effect on silicosis is significant and superior to conventional immunosuppressive and cytotoxic drugs.
References
Gralla, Coleman, Jonas, Cancer Chemother. Rep., Pt. 3, 5(1), 79 (1974)
D-Tetrandrine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12835 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5858 | 58 |
| Watson Biotechnology Co.,Ltd | +86-18186686046 +86-18186686046 | sales01@watsonbiotech.cn | China | 5820 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21635 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29885 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3772 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 | sales@czwytech.com | CHINA | 904 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
View Lastest Price from D-Tetrandrine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-13 | D-Tetrandrine
518-34-3
|
US $0.00-0.00 / G | 100G | 98% | 50KG | Watson Biotechnology Co.,Ltd | |
 |
2024-11-13 | Stephania Tetrandra extract Tetrandrine
518-34-3
|
US $0.00-0.00 / kg | 1kg | 98% | 1000kg | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-11-11 | Tetrandrine
518-34-3
|
US $89.00-43.00 / mg | 99.72% | 10g | TargetMol Chemicals Inc. |
-

- D-Tetrandrine
518-34-3
- US $0.00-0.00 / G
- 98%
- Watson Biotechnology Co.,Ltd
-

- Stephania Tetrandra extract Tetrandrine
518-34-3
- US $0.00-0.00 / kg
- 98%
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- Tetrandrine
518-34-3
- US $89.00-43.00 / mg
- 99.72%
- TargetMol Chemicals Inc.
518-34-3(D-Tetrandrine)Related Search:
1of4