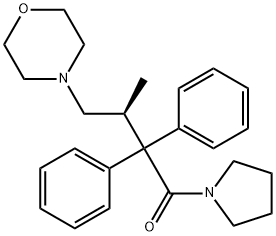
dextromoramide
- CAS No.
- 357-56-2
- Chemical Name:
- dextromoramide
- Synonyms
- Pyrrolamidol;dextromoramide;(+)-1-[(S)-3-Methyl-4-morpholino-2,2-diphenylbutyryl]pyrrolidine;1-Butanone, 3-methyl-4-(4-morpholinyl)-2,2-diphenyl-1-(1-pyrrolidinyl)-, (3S)-
- CBNumber:
- CB9906147
- Molecular Formula:
- C25H32N2O2
- Molecular Weight:
- 392.53
- MDL Number:
- MOL File:
- 357-56-2.mol
| Melting point | 180-184° |
|---|---|
| alpha | D20 +25.5° (c = 5 in benzene) |
| Boiling point | 526.13°C (rough estimate) |
| Density | 1.1004 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| solubility | Benzene (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| form | Thick Oil |
| pka | pKa 7.0 (Uncertain) |
| color | Colourless to Pale Yellow |
| FDA UNII | 9S4S6CIY83 |
| ATC code | N02AC01 |
dextromoramide Chemical Properties,Uses,Production
Originator
Palfium,ACE Pharmaceuticals B.V.
Uses
(3S)-3-Methyl-4-(4-morpholinyl)-2,2-diphenyl-1-(1-pyrrolidinyl)-1-butanone is an intermediate in the synthesis of (S)-Moramide or Dextromoramide (M630190), which is a controlled substance (opiate). Analgesic (narcotic).
Definition
ChEBI: An N-acylpyrrolidine arising by formal condensation of pyrrolidine with (3S)-3-methyl-4-(morpholin-4-yl)-2,2-diphenylbutanoic acid. An opioid analgesic that is structurally related to methadone, it is more poweful than m rphine but shorter acting. It has been used (particularly as the hydrogen tartrate salt) for the treatment of severe pain, but was discontinued in the UK in 2004.
Manufacturing Process
1 mol diphenylacetylpyrrolidine was added to a suspension of 1,1-1,3 mol sodium oxide in 700 ml of toluene. The mixture was refluxed for a few hours until the sodium compounds of the acetamide derivative were formed completely. Then a slight excess of 1-(2-chloropropyl)morpholine was slowly added, dissolved in an equal volume of toluene or xylene. This mixture was refluxed for 6-8 hours. The reaction mixture was treated with water. The organic layer was then extracted with dilute hydrochloric acid and this extract was again made alkaline and extracted with ether. The extract was dried with potassium carbonate. A small amount of diphenylacetylpyrrolidine crystallizes from the ether. This is filtered off, petroleum ether is added and mixture is placed in refrigerator. After some days the 1-(4-N-morpholino-4-methyl-2,2- diphenylbutyryl)pyrrolidine is separated from this extract. M.P. 109-111°C.
brand name
Dimorlin Tartrate (SmithKline Beecham).
Therapeutic Function
Narcotic analgesic