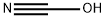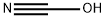cyanic acid
- CAS No.
- 420-05-3
- Chemical Name:
- cyanic acid
- Synonyms
- CPD-69;cyanicaci;cyanic acid;hydrogen cyanate;EVONIK VESTANAT B1042E;Cyanic acid (6CI,7CI,8CI,9CI)
- CBNumber:
- CB9945465
- Molecular Formula:
- CHNO
Lewis structure

- Molecular Weight:
- 43.02
- MDL Number:
- MFCD00871274
- MOL File:
- 420-05-3.mol
| Melting point | -86° |
|---|---|
| Boiling point | bp760 23.5° |
| Density | d420 1.140; d4-20 1.156 |
| refractive index | 1.4300 (estimate) |
| pka | 3.7(at 25℃) |
| Exposure limits | No exposure limit has yet been set for this compound. |
| FDA UNII | 460E3FHT8O |
| EPA Substance Registry System | Cyanic acid (420-05-3) |
cyanic acid Chemical Properties,Uses,Production
Preparation
Cyanic acid is prepared in the laboratory by dry distillation of cyanuric acid, C3N3(OH)3.
Reaction
Cyanic acid decomposes on heating. Rapid heating may cause explosion. When heated to high temperatures, it decomposes forming carbon dioxide, water, and nitrogen oxides:
4NCOH + 7O2 4CO2 + 4NO2 + 2H2O
It dissolves in water decomposing to carbon dioxide and ammonia. Although the reaction occurs at ordinary temperatures, it is slow in dilute aqueous solutions at ice temperature.
NCOH + H2O → CO2 + NH3
The compound polymerizes on standing, forming cyanuric acid, an oxygen heterocylic compound, 1,3,5-trioxane-2,4,6-triimine, C3H3N3O3.
Chemical Properties
Colorless liquid or gas with an acrid smell; mp -86°C (-122.8°F); bp 23.5°C (74.3°F); density 1.140 at 20°C (68°F); soluble in water, alcohol, ether, benzene, and toluene.
Uses
Hydrogen cyanate is used in the preparation of cyanates.
Uses
In formation of some cyanates.
Definition
cyanic acid: An unstable explosiveacid, HOCN. The compound has thestructure H–O–C≡N, and is also calledfulminic acid. Its salts and esters arecyanates (or fulminates). The compoundis a volatile liquid, whichreadily polymerizes. In water it hydrolysesto ammonia and carbondioxide. It is isomeric with anotheracid, H–N=C=O, which is known asisocyanic acid. Its salts and esters areisocyanates.
Health Hazard
Hydrogen cyanate is a severe irritant to the eyes, skin, and mucous membranes. Exposure to this compound can cause severe lacrimation. Inhalation can produce irritation and injury to the respiratory tract. LD50 values are not reported.
Fire Hazard
Flammable; the liquid can explode when heated rapidly.
Waste Disposal
Hydrogen cyanate can be disposed of in the drain in small amounts. It decomposes in water forming CO2 and NH3.
cyanic acid Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +86-15271838296; +8615271838296 | kyra@quanjinci.com | China | 1512 | 58 |
| Hubei Zhonglong Kangsheng fine chemical Co.,Ltd | 027-83850299 15827297403 | 2315383731@qq.com | China | 4281 | 58 |
| Supplier | Advantage |
|---|---|
| Shaanxi Didu New Materials Co. Ltd | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | 58 |
| Hubei Zhonglong Kangsheng fine chemical Co.,Ltd | 58 |
420-05-3(cyanic acid)Related Search:
1of4