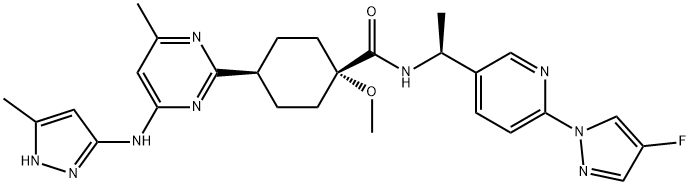
Pralsetinib
- CAS No.
- 2097132-94-8
- Chemical Name:
- Pralsetinib
- Synonyms
- PRALSETINIB;BLU-667;Rralsetinib;CPD2049;BLU-667-API;13C4]-Pralsetinib;PRALSETINIB (BLU667);BLU-667 (Pralsetinib);Pralsetinib Monohydrate;BLU-667;BLU 667;BLU667;2097132-94-8
- CBNumber:
- CB04632289
- Molecular Formula:
- C27H32FN9O2
- Molecular Weight:
- 533.6
- MOL File:
- 2097132-94-8.mol
- Modify Date:
- 2024/7/24 13:33:21
| Boiling point | 799.1±60.0 °C(Predicted) |
|---|---|
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Store at 4°C |
| solubility | DMSO : ≥ 100 mg/mL (187.41 mM);Water : < 0.1 mg/mL (insoluble) |
| form | Solid |
| pka | 14.33±0.10(Predicted) |
| color | White to off-white |
| InChIKey | GBLBJPZSROAGMF-BATDWUPUSA-N |
| SMILES | [C@]1(OC)(C(N[C@H](C2=CC=C(N3C=C(F)C=N3)N=C2)C)=O)CC[C@H](C2=NC(NC3C=C(C)NN=3)=CC(C)=N2)CC1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
Pralsetinib Chemical Properties,Uses,Production
Description
Pralsetinib is a potent, selective RET inhibitor, and was optimized from a hit compound identified from a compound library that included more than 60 chemical scaffolds. However, as of 2020, detailed medicinal chemistry on the optimization process has yet to be published. Pralsetinib proved to be more potent and selective than cabozantinib or vandertanib against both wild-type and abnormal RET. The agent also showed a level of selectivity against RET relative to VEGFR2, whereas previous agents showed very little selectivity. On September 4, 2020, the Food and Drug Administration granted accelerated approval to pralsetinib (GAVRETO®, Blueprint Medicines Corporation) for adult patients with metastatic RET fusion-positive non-small cell lung cancer (NSCLC) as detected by an FDA-approved test.
Uses
Pralsetinib is a highly potent and selective RET inhibitor designed for RET-driven cancers.
Indications
Pralsetinib is the second selective RET inhibitor approved by the FDA (following Lilly's selpercatinib) for the treatment of adult patients with metastatic RET (rearranged during transfection) fusion-positive non-small cell lung cancer (NSCLC); (2) adult and pediatric patients >=12 years of age with advanced or metastatic RET-mutant medullary thyroid cancer (MTC) who require systemic therapy; and (3) adult and pediatric patients >=12 years of age with advanced or metastatic RET fusion-positive thyroid cancer who require systemic therapy and who are radioactive iodine-refractory.
brand name
Gavreto
Synthesis Reference(s)
[1] GAIKWAD RAJENDRA. Green One-Pot Chemo-Enzymatic Synthesis of a Key Chiral Amine Intermediate: Useful to Pralsetinib Synthesis[J]. ChemistrySelect, 2023. DOI:10.1002/slct.202204409.
[2] HUGHES* D L. Review of Synthetic Routes and Crystalline Forms of the Oncology Drugs Capmatinib, Selpercatinib, and Pralsetinib[J]. Organic Process Research & Development, 2021. DOI:10.1021/acs.oprd.1c00282.
General Description
Class: receptor tyrosine kinase; Treatment: RET-altered lung, thyroid cancers; Other name: BLU-667; Elimination half-life = 22 h; Protein binding = 97%
Side effects
Pralsetinib may cause side effects: Nausea, vomiting, loss of appetite, diarrhea, constipation, extreme tiredness, dizziness, weakness, night sweats, rapid heartbeat, heartburn, shortness of breath, headache, sores in the mouth, confusion, changes in vision, fever, cough, chills, nosebleeds, pale skin, rash, itching, hives, weight changes, hair loss, back pain, muscle pain, joint pain, bone pain, unusual bruising or bleeding, blood in the urine or stool, bloody or black tarry stools, difficulty falling asleep or staying asleep, unusual vaginal bleeding, and other less common effects.
target
Primary target: RET
Mode of action
Kinase inhibitor of wild-type RET, oncogenic RET fusions, and select mutations. Pralsetinib may also inhibit other pathways including those through FLT3, JAK1-2, PDGFRB, VEGFR-2, and FGFR1. RET fusion proteins and activating point mutations can act as oncogenic drivers by promoting cell proliferation of tumor cell lines and pralsetinib inhibits this process.
Pralsetinib Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | China | 3136 | 58 | Inquiry |
| Wuhan Jingkang en Biomedical Technology Co., Ltd | +8613720134139 | China | 5225 | 58 | Inquiry |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | China | 227 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 18642 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32836 | 60 | Inquiry |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | China | 10311 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Chengdu Aslee Biopharmaceuticals, Inc. | 28-85305008 | CHINA | 964 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30250 | 58 | Inquiry |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | China | 10522 | 58 | Inquiry |
Related articles
- Pralsetinib: Indications, Mechanism of action and Side Effects
- Pralsetinib is a kinase inhibitor that targets wild-type RET and kinase-activated RET mutations and fusions.
- Jun 25,2024
- Pralsetinib: Synthesis and Introduction
- Pralsetinib (BLU-667) is synthesised using bromopyridine as a raw material by chemical reaction.
- Jan 2,2024





