Carbamazepine
- CAS No.
- 298-46-4
- Chemical Name:
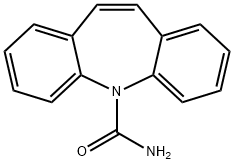
- Carbamazepine
- Synonyms
- CARBAMAZEPIN;5H-Dibenzo[b,f]azepine-5-carboxamide;TEGRETOL;Epitol;Finlepsin;KAMAXIPING;Carbamezepine;Oxcarbazepine IMpurity A;Lexin;Biston
- CBNumber:
- CB1143564
- Molecular Formula:
- C15H12N2O
- Molecular Weight:
- 236.27
- MOL File:
- 298-46-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 191-192 °C (lit.) |
|---|---|
| Boiling point | 378.73°C (rough estimate) |
| Density | 1.1099 (rough estimate) |
| refractive index | 1.5906 (estimate) |
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: soluble29mg/mL |
| pka | 13.94±0.20(Predicted) |
| form | Crystals |
| color | Almost white |
| Water Solubility | pract. insoluble |
| Merck | 14,1781 |
| BCS Class | 2 |
| LogP | 2.450 |
| CAS DataBase Reference | 298-46-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Carbamazepine(298-46-4) |
| EPA Substance Registry System | 5H-Dibenz[b,f]azepine-5-carboxamide (298-46-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H317-H336-H360D | |||||||||
| Precautionary statements | P201-P280-P301+P312+P330-P302+P352-P308+P313 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 42/43-22-20/21/22-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 37-24-22-36/37/39-36-45-36/37-16 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | HN8225000 | |||||||||
| HS Code | 29339900 | |||||||||
| Hazardous Substances Data | 298-46-4(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in mice, rats: 3750, 4025 mg/kg (Stenger, Roulet) | |||||||||
| NFPA 704 |
|
Carbamazepine price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | C4024 | Carbamazepine powder | 298-46-4 | 1G | ₹3063.48 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C8981 | Carbamazepine meets USP testing specifications | 298-46-4 | 5G | ₹7609.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1067 | Carbamazepine Pharmaceutical Secondary Standard; Certified Reference Material | 298-46-4 | 1G | ₹8465.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C4024 | Carbamazepine powder | 298-46-4 | 5G | ₹8151.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C8981 | Carbamazepine meets USP testing specifications | 298-46-4 | 25G | ₹21227.83 | 2022-06-14 | Buy |
Carbamazepine Chemical Properties,Uses,Production
Description
Carbamazepine is a synthetic iminostilbene derivative structurally similar to imipramine, a tricyclic antidepressant. While unrelated structurally, carbamazepine shares a similar therapeutic action with phenytoin. Carbamazepine was first discovered in 1953 by Swiss chemist Walter Schindler. Throughout the 1960s, antimuscarinic was used and marketed for trigeminal neuralgia and as an anticonvulsant. By the 1970s, it was being used as a mood stabilizer for patients with bipolar disorder.
Chemical Properties
White or off-white crystalline powder. Soluble in ethanol, acetone, propylene glycol, insoluble in water. Odorless and tasteless.
Uses
Carbamazepine (CBZ) is a first generation anticonvulsant and mood stabilizing compound that has been used as a therapeutic in the context of neuropathic pain, epilepsy, and affective disorders. It exerts its effects by blocking voltage-gated sodium channels (IC50 = 640 μM), making fewer of these channels available to subsequently open, which leads to decreased high-frequency repetitive firing of action potentials. The estimated IC50 values for inhibition of Nav1.7-, Nav1.3-, and Nav1.8-type channels by CBZ following prolonged inactivation have been reported as 406, 900, and 138 μM, respectively. CBZ can also inhibit L-type Ca2+ channels (IC50 = 974 μM) and has been shown to potentiate GABAA receptors (IC50 >3 mM).
Definition
ChEBI: Carbamazepine is a dibenzoazepine that is 5H-dibenzo[b,f]azepine carrying a carbamoyl substituent at the azepine nitrogen, used as an anticonvulsant. It has a role as an anticonvulsant, an EC 3.5.1.98 (histone deacetylase) inhibitor, a mitogen, a glutamate transporter activator, an antimanic drug, an analgesic, a non-narcotic analgesic, an environmental contaminant, a xenobiotic, a drug allergen and a sodium channel blocker. It is a dibenzoazepine and a member of ureas.
Biological Functions
Carbamazepine has become a major drug in the treatment
of seizure disorders. It has high efficacy, is well tolerated
by most patients, and exhibits fewer long-term
side effects than other drugs.
Oral absorption of carbamazepine is quite slow and
often erratic. Its half-life is reported to vary from 12 to
60 hours in humans.The development of blood level assays
has markedly improved the success of therapy with
this drug, since serum concentration is only partially
dose related. Carbamazepine is metabolized in the liver,
and there is evidence that its continued administration
leads to hepatic enzyme induction. Carbamazepine-
10,11-epoxide is a pharmacologically active metabolite with significant anticonvulsant effects of its own.
General Description
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards.
Carbamazepine is a tricyclic lipophilic compound, with mild anticholinergic activity. It is widely used as an antiepileptic drug for the treatment of simple and complex partial tonic-clonic seizures.
Mechanism of action
In animals, the profile of antiseizure properties for CBZ is similar to that of phenytoin. CBZ is effective in the maximal electroshock (MES) test (electrically induced seizure test) but is ineffective against pentylenetetrazole-induced seizures. It is not effective for absence or myoclonic seizures and, indeed, may exacerbate their onset. Like phenytoin, CBZ acts on voltage-dependent sodium channels to prevent the spread of seizures. CBZ depresses synaptic transmission in the reticular activating system, thalamus, and limbic structures. In a double-blind, crossover study in patients whose seizures were not controlled completely by combinations of AED, CBZ was equal in efficacy to phenobarbital and phenytoin in controlling seizure frequency, and side effects were minimal.
Clinical Use
Carbamazepine is an effective agent for the treatment of partial seizures and generalized tonic–clonic seizures; its use is contraindicated in absence epilepsy. Carbamazepine is also useful in the treatment of trigeminal neuralgia and is an effective agent for the treatment of bipolar disorders.
Drug interactions
Carbamazepine may be affected by other medicines, such as blood clot preventers and antibiotics. It may also interact with medications used to treat bipolar disorder. Some medications can decrease carbamazepine levels and others can increase carbamazepine levels. Valproate may increase carbamazepine-10,11-epoxide levels, while carbamazepine may decrease the levels of various medications including corticosteroids, clozapine, and lamotrigine. It is important to inform your doctor if you are taking any of these medicines to avoid potential interactions and to ensure carbamazepine works effectively.
Taking carbamazepine with other medicines and herbal supplements
What drug-drug interactions should you be vigilant for when someone is prescribed carbamazepine
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c13bc0b8-7900-4ef4-98ed-e1315a08d95d
Environmental Fate
Carbamazepine is both an important anticonvulsant in therapeutic doses and a powerful proconvulsant in overdose. The therapeutic anticonvulsant mechanism is primarily related to blockade of presynaptic voltage-gated sodium channels. Blockade of the sodium channels is believed to inhibit the release of synaptic glutamate and possibly other neurotransmitters. Carbamazepine is also a powerful inhibitor of the muscarinic and nicotinic acetylcholine receptors, N-methyl-Daspartate (NMDA) receptors, and the central nervous system (CNS) adenosine receptors. In addition, carbamazepine is structurally related to the cyclic antidepressant imipramine and in massive overdose, it may affect cardiac sodium channels.
Carbamazepine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TAGOOR LABORATORIES PVT LTD | +919700187008 | AndhraPradesh, India | 90 | 58 | Inquiry |
| Halcyon Labs Pvt. Ltd | +919833750169 | Mumbai, India | 17 | 58 | Inquiry |
| CLEANCHEM LABORATORIES LLP | +91-9951015911 +91-9951015911 | Mumbai, India | 281 | 58 | Inquiry |
| Frolic Pharmachem | +919711094561 | Himachal Pradesh, India | 94 | 58 | Inquiry |
| WARUKSHA LABORATORIES PVT LTD | +91-7485988764 +91-7485988764 | Gujarat, India | 77 | 58 | Inquiry |
| Global Pharma | +91-9819994077 +91-9819994077 | Maharashtra, India | 34 | 58 | Inquiry |
| Shree HariKrishna Pharmaceuticals | +91-9925611910 +91-9925611910 | Gujarat, India | 16 | 58 | Inquiry |
| Bajaj Healthcare Ltd | +91-2266177400 +91-2266177400 | Maharashtra, India | 86 | 58 | Inquiry |
| Venture Pharmaceuticals pvt ltd | +91-9173909075 +91-9173909075 | Gujarat, India | 45 | 58 | Inquiry |
| CTX Lifesciences Pvt Ltd | +91-2612399669 +91-7434851395 | Gujarat, India | 28 | 58 | Inquiry |
Related articles
- Carbamazepine: Indications, Dose, Mechanism of action and Side Effects
- Carbamazepine is a commonly used antiepileptic drug. Its FDA-approved indications include: Epilepsy, Trigeminal neuralgia, tic....
- Aug 23,2024
- Carbamazepine:pharmacokinetics, clinical efficacy, and side effects
- Carbamazepine is a widely used antiepileptic drug that offers clinicians many choices for treating epilepsy and trigeminal neu....
- Jun 7,2023
- Uses of Carbamazepine
- Carbamazepine is a synthetic iminostilbene derivative structurally similar to imipramine, a tricyclic antidepressant. While un....
- Dec 8,2021
298-46-4(Carbamazepine)Related Search:
1of4
chevron_right




