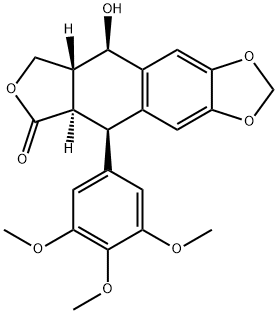
Podophyllotoxin
- CAS No.
- 518-28-5
- Chemical Name:
- Podophyllotoxin
- Synonyms
- podofilox;PODOPHYLLIC ACID LACTONE;condylox;Condyline;β-Arteether;(-)-PODOPHYLLOTOXIN;nsc24818;518-28-5;(+)-Shikonin;(+)-Arteether
- CBNumber:
- CB1147810
- Molecular Formula:
- C22H22O8
- Molecular Weight:
- 414.41
- MOL File:
- 518-28-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/11 18:14:38
| Melting point | 183-184 °C (lit.) |
|---|---|
| alpha | -110.7 º (c=1, CHCl3) |
| Boiling point | 453.31°C (rough estimate) |
| Density | 1.2649 (rough estimate) |
| refractive index | 1.4480 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO:15.0(Max Conc. mg/mL);36.2(Max Conc. mM) |
| form | Powder |
| pka | 13.26±0.40(Predicted) |
| color | White to off-white |
| optical activity | [α]/D 131±2°, c = 1 in chloroform |
| Merck | 13,7628 |
| BRN | 99163 |
| Stability | Very Hygroscopic |
| InChIKey | YJGVMLPVUAXIQN-XVVDYKMHSA-N |
| LogP | 2.010 |
| CAS DataBase Reference | 518-28-5(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280-P301+P310-P302+P352+P312-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 21-25-36/37/38-23/25-23/24/25 | |||||||||
| Safety Statements | 36/37/39-45-26 | |||||||||
| RIDADR | UN 3462 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LV2500000 | |||||||||
| F | 1-8-10 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29189090 | |||||||||
| Toxicity | LD50 in rats (mg/kg): 8.7 i.v.; 15 i.p. (Phillips) | |||||||||
| NFPA 704 |
|
Podophyllotoxin price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | P4405 | Podophyllotoxin | 518-28-5 | 50MG | ₹5531.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P4405 | Podophyllotoxin | 518-28-5 | 100MG | ₹9255.38 | 2022-06-14 | Buy |
| TCI Chemicals (India) | P1771 | Podophyllotoxin | 518-28-5 | 1G | ₹3500 | 2022-05-26 | Buy |
Podophyllotoxin Chemical Properties,Uses,Production
Chemical Properties
off-white fine crystalline powder
History
Podophyllotoxin was first found in the Podophyllum peltatum L.?The first time to
isolate podophyllotoxin from podophyllin was in 1880. In 1942, it was found that
venereal warts could be effectively treated by application of podophyllin.Subsequently, podophyllotoxin was reported to inhibit the growth of the tumor
through the inhibition of the microtubule formation. The chemical structure of
podophyllotoxin was elucidated in 1951.
In the 1960s, two main podophyllotoxin derivatives were synthesized, etoposide
and teniposide (VM-26) . In 1983, etoposide was approved by FDA.?Etoposide
and teniposide are used in frontline cancer therapy against various cancer types,
such as small cell lung cancer, testicular cancer, etc. In 1996, etoposide phosphate
analog (Etopophos) was launched in America. Etopophos is the prodrug of etoposide and can be rapidly absorbed and completely converted to the parent compound
in?vivo. In 1990, WHO recommended 0.5% podophyllotoxin as the first-line drug
for the treatment of condyloma acuminatum. Podophyllotoxin creams and gels are
nowadays widely used in clinical practice.
Uses
Podophyllotoxin is a non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum species. It binds to topoisomerase II during the late S and early G2 stage, blocking tubulin polymerization and, thus, inhibiting mitosis. In addition to being used as a cathartic, purgative, antiviral agent, vesicant, and antihelminthic, podophyllotoxin is the starting material for the semi-synthesis of the anti-cancer drugs etoposide , teniposide , and etopophos.
Indications
Podophyllotoxin (Podofilox) is available alone and as the main cytotoxic ingredient in podophyllin (25% podophyllum resin), a mixture of toxic chemicals derived from May apple plants. The active ingredients inhibit cell mitosis. The drugs are used to treat condylomata acuminata. The most common toxic effects are skin irritation and less commonly, ulceration. Systemic absorption of podophyllin can occur (especially if applied to large, inflamed areas or mucosal surfaces), with gastrointestinal, hematological, renal, and hepatotoxic effects. In addition, seizures and peripheral neuropathy have been reported.
Definition
ChEBI: An organic heterotetracyclic compound that has a furonaphthodioxole skeleton bearing a 3,4,5-trimethoxyphenyl substituent. It is found in the roots and rhizomes of Podophyllum species and is used for the topical treatment of genital warts.
Pharmacology
Antineoplastic and antiviral activities are the most pronounced pharmacological. effects of podophyllotoxin . Podophyllotoxin shows a significant inhibitory effect on the division and proliferation of epithelial cells infected by human papillomavirus (HPV), disrupts the cell cytoskeleton, and induces the necrosis and shedding of warts. It was shown that the antitumor effect of podophyllotoxin is associated with the inhibition of microtubule assembly and the induction of apoptosis. However, the antitumor effect of podophyllotoxin analogs, such as etoposide, teniposide, and Etopophos, is related to disparate mechanisms including the inhibition of DNA topoisomerase II activity and the formation of stable nucleic acid-drugenzyme complex, which induce DNA double-strand or single-strand break and eventually lead to cell death . It was also found that podophyllotoxin derivatives have immunosuppressive and anti-inflammatory effects.
Clinical Use
Podophyllotoxin is a useful agent for the treatment of condyloma acuminatum . Podophyllotoxin and its derivatives are also widely used in the treatment of cancer, such as lymphomas and lung carcinoma. Because of the several toxicity of podophyllotoxin, for example, the irritation of skin and mucous membranes, combination therapies are used to treat condyloma acuminatum or cancer.
Anticancer Research
Podophyllotoxin (PTOX) is an aryl-tetralin lignan and has been originallyisolated from Podophyllum peltatum L. (American podophyllum or Mayapple;family Podophyllaceae). Later, it is also isolated from several species like P.hexandrum Royle (Indian podophyllum) and P. pleianthum (Taiwanese podophyllum).PTOX has also been reported in other plants such as Linum spp., Callitrisspp., Juniperus spp., Thuja spp., Hyptis spp., Thymus spp., Teucrium spp., Nepetaspp., Dysosma spp., Diphylleia spp., and Jeffersoniana spp. (Ionkova 2007;Yousefzadi et al. 2010). PTOX shows strong cytotoxic activity against various cancercell lines. However, PTOX is too toxic for the treatment of neoplastic diseasesin humans; it is used as a precursor for chemical synthesis of semisynthetic antineoplasticdrugs, etoposide, Etopophos, and teniposide , which are successfullyused as antitumor agents (Holthuis 1988; Cragg and Newman 2005).Podophyllotoxin derivatives are used in the treatment of lymphomas, acute leukemia,and testicular, lung, ovarian, bladder, and brain cancer (Srivastava et al. 2005).Podophyllum spp. are the major source of PTOX, and their availability is limited innature, and some species are categorized as endangered. Moreover, the chemicalsynthesis of podophyllotoxin is an expensive process; therefore, biotechnologicalproduction of podophyllotoxin using plant cell and tissue cultures has been preferredby various research groups (Farkya et al. 2004).
Purification Methods
The toxin recrystallises form *C6H6 (with 0.5C6H6), EtOH/*C6H6, aqueous EtOH (with 1-1.5H2O, m 114-115o) and CH2Cl2/pentane. When dried at 100o/10mm it has m 183-184o. [UV: Stoll et al. Helv Chim Acta 37 1747 1954, IR: Schecler et al. J Org Chem 21 288 1956.] It is an inhibitor of microtubule assembly [Prasad et al. Biochemistry 25 739 1986]. [Beilstein 19/10 V 666.]
Podophyllotoxin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| Himpharm | +91-9816648055 +91-9816664455 | Himachal Pradesh, India | 29 | 58 | Inquiry |
| Green Vision Life Sciences Pvt Ltd. | +91-2067919200 +91-9404953243 | Maharashtra, India | 30 | 58 | Inquiry |
| Inga Pharmaceuticals | +91-2228202932 +91-2228202932 | Maharashtra, India | 38 | 58 | Inquiry |
| Namiex Chemicals Private Limited | +91-1862225256 +91-9988625256 | Punjab, India | 13 | 58 | Inquiry |
| Synnat Pharma Pvt Ltd | +91-9966980001 +91-9989984964 | AndhraPradesh, India | 22 | 58 | Inquiry |
| Sarv Bio Labs Pvt Ltd | +91-9958217221 +91-9875997034 | Haryana, India | 10 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
518-28-5(Podophyllotoxin)Related Search:
1of4
chevron_right




