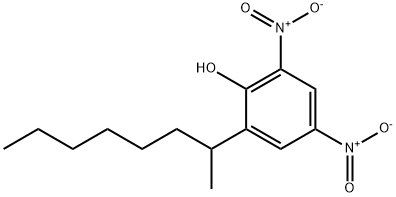
2,4-dinitro-6-(1-methylheptyl)phenol
- CAS No.
- 3687-22-7
- Chemical Name:
- 2,4-dinitro-6-(1-methylheptyl)phenol
- Synonyms
- Clonidine Impurity 16;DNOP (technical mixture);2,4-dinitro-6-(oct-2-yl)phenol;2,4-Dinitro-6-(octan-2-yl)phenol;2,4-dinitro-6-(1-methylheptyl)phenol;Phenol, 2-(1-methylheptyl)-4,6-dinitro-;2,4-Dinitro-6-(octan-2-yl)phenol Solution, 100ppm;2,4-Dinitro-6-(octan-2-yl)phenol Solution in Acetonitrile, 100μg/mL
- CBNumber:
- CB12470882
- Molecular Formula:
- C14H20N2O5
- Molecular Weight:
- 296.32
- MOL File:
- 3687-22-7.mol
- Modify Date:
- 2023/5/15 10:43:59
| Density | 1.16 at 21℃ |
|---|---|
| vapor pressure | 0.005Pa at 25℃ |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| color | Light Yellow to Yellow |
| Stability | Stability |
| LogP | 6.27 |
| Dissociation constant | 5.69-6.79 at 25℃ |
| CAS DataBase Reference | 3687-22-7 |
| EPA Substance Registry System | 2,4-Dinitro-6-(1-methylheptyl)phenol (3687-22-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS09,GHS05,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H410-H315-H301-H317-H318 |
| Precautionary statements | P273-P391-P501-P264-P280-P302+P352-P321-P332+P313-P362-P264-P270-P301+P310-P321-P330-P405-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P280-P305+P351+P338-P310 |
2,4-dinitro-6-(1-methylheptyl)phenol Chemical Properties,Uses,Production
Reactivity Profile
2,4-dinitro-6-(1-methylheptyl)phenol is an organonitrate/phenol. Organonitrate compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases.
Fire Hazard
Flash point data for 2,4-dinitro-6-(1-methylheptyl)phenol are not available. 2,4-dinitro-6-(1-methylheptyl)phenol is probably combustible.
2,4-dinitro-6-(1-methylheptyl)phenol Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Cochemical Co. Ltd. | +86-029-86115547 +86-17791676824 | China | 3953 | 58 | Inquiry |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 | China | 56077 | 58 | Inquiry |
| Shanghai Hao Zhun Biological Technology Co., Ltd. | 15800340161 | CHINA | 6846 | 58 | Inquiry |
| Alta Scientific Co., Ltd. | 022-6537-8550 15522853686 | China | 4515 | 55 | Inquiry |
| TCI (Shanghai) Chemical Trading Co., Ltd. | 021-021-61109150 | China | 31130 | 58 | Inquiry |
| Qingzhou dafeng import and export Co. LTD | 16773308882 | China | 3051 | 58 | Inquiry |
| Shanghai Saikerui Biotechnology Co. , Ltd. | 021-58000709 15900491054 | China | 9268 | 58 | Inquiry |





