ChemicalBook > Product Catalog >Biochemical Engineering >Amino Acids and Derivatives >Fmoc-N-Me-Lys(Boc)-OH
Fmoc-N-Me-Lys(Boc)-OH
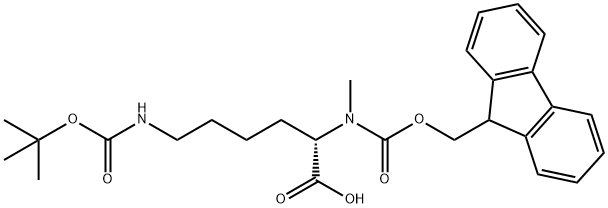
- CAS No.
- 197632-76-1
- Chemical Name:
- Fmoc-N-Me-Lys(Boc)-OH
- Synonyms
- FM;FM;FMOC-MELYS(BOC)-OH;FMOC-L-MELYS(BOC)-OH;FMOC-N-ME-LYS(BOC)-OH;FMoc-Nw-Me- Lys(Boc)-OH;FMOC-N-ME-LYS(BOC)-OH 1 G;Fmoc-Nω-Me-Lys(Boc)-OH, >97%;FMOC-N-ME-LYS(BOC)-OH 250 MG;N'-Cbz-N-Fmoc-N-methyl-L-lysine
- CBNumber:
- CB1473656
- Molecular Formula:
- C27H34N2O6
- Molecular Weight:
- 482.57
- MOL File:
- 197632-76-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:01
| Melting point | 189-194°C (decomposition) |
|---|---|
| Boiling point | 665.4±55.0 °C(Predicted) |
| Density | 1.200 |
| storage temp. | 2-8°C |
| form | Solid |
| pka | 3.90±0.21(Predicted) |
| color | White to off-white |
| optical activity | [α]22/D -14.0°, c = 1% in chloroform |
| InChIKey | JMBKBGOKNZZJQA-QHCPKHFHSA-N |
| CAS DataBase Reference | 197632-76-1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| WGK Germany | 3 |
| HazardClass | IRRITANT |
| HS Code | 29242990 |
Fmoc-N-Me-Lys(Boc)-OH price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.52361 | Fmoc-N-Me-Lys(Boc)-OH Novabiochem? | 197632-76-1 | 250MG | ₹16460 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.52361 | Fmoc-N-Me-Lys(Boc)-OH Novabiochem? | 197632-76-1 | 1G | ₹49640 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 773107 | Fmoc-N-Me-Lys(Boc)-OH 97% (HPLC) | 197632-76-1 | 250MG | ₹12110 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 773107 | Fmoc-N-Me-Lys(Boc)-OH 97% (HPLC) | 197632-76-1 | 1G | ₹31840 | 2022-06-14 | Buy |
Fmoc-N-Me-Lys(Boc)-OH Chemical Properties,Uses,Production
Description
Fmoc-N-Me-Lys(Boc)-OH is a peptide that mimics the structure of the natural substrate of serine protease. It has been shown to be stable in extracellular fluids and resistant to proteolytic degradation. The systematic sequence was designed to maximize the chance of binding to the active site of serine proteases while minimizing binding to other proteases. The maximal modifications were made on the amino acid side chains, which are most likely to interact with other proteins or with cell surfaces.
Chemical Properties
white to slight yellow to beige
Fmoc-N-Me-Lys(Boc)-OH Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 100)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Sichuan HongRi Pharma-Tech Co.,Ltd | +86-028-64841719 +8617390183901 | China | 1381 | 58 | Inquiry |
| Alpha Biopharmaceuticals Co., Ltd | +86-15542445688 | China | 888 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9337 | 55 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Apeloa Pharmaceutical Co., Ltd. | +86-0571-87635730 +8615858229168 | China | 1478 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
197632-76-1(Fmoc-N-Me-Lys(Boc)-OH )Related Search:
N-Methyl-1-naphthalenemethylamine hydrochloride
FMOC-N-METHYL-L-NORLEUCINE
Fmoc-n-amido-peg10-acid
Fmoc-S-trityl-alpha-Me-L-cysteine
FMOC-N-Methyl-L-alanine
Fmoc-N-Me-Arg(pbf)-OH
FMoc-N-Me-Lys(2-Cl-Z)-OH
Boc-N-Me-Lys(N3)-OH
Boc-N-Me-Lys(Fmoc)-OH
Boc-N-Me-Lys(2-Cl-Z)-OH
chevron_left
1of4
chevron_right
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-N-ALPHA-METHYL-N-EPSILON-T-BUTYL-OXYCARBONYL-L-LYSINE
N-ALPHA-(9-FLUORENYLMETHYLOXYCARBONYL)-N-ALPHA-METHYL-N-EPSILON-TERT-BUTYLOXYCARBONYL-L-LYSINE
FMOC-N-ALPHA-METHYL-N-EPSILON-T-BOC-L-LYSINE
FMOC-MELYS(BOC)-OH
FMOC-N-ME-LYS(BOC)-OH
FMOC-L-MELYS(BOC)-OH
Fmoc-Nalpha-methyl-Ne-t-Boc-L-lysine
N'-[(tert-Butoxy)carbonyl]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N-methyl-L-lysine
N6-[(1,1-Dimethylethoxy)carbonyl]-N2-[(9H-fluoren-9-ylmethoxy)carbonyl]-N2-methyl-L-lysine
N'-Cbz-N-Fmoc-N-methyl-L-lysine
NA-FMOC-NA-METHYL-NE-BOC-L-LYSINE
FMoc-Nw-Me- Lys(Boc)-OH
N^e-Boc-N^a-FMoc-N^a-Methyl-L-lysine, 98%
Nα-Fmoc-Nα-methyl-Nε-t-Boc-L-lysine
Nα-FMoc-Nα-Methyl-Nε-Boc-L-lysine
(S)-2-((((9H-Fluoren-9-yl)Methoxy)carbonyl)(Methyl)aMino)-6-((tert-butoxycarbonyl)aMino)hexanoic acid
N'-Boc-N-Fmoc-N-methyl-L-lysine
N2-(((9H-Fluoren-9-yl)methoxy)carbonyl)-N6-(tert-butoxycarbonyl)-N2-methyl-L-lysine
(2S)-6-{[(tert-butoxy)carbonyl]amino}-2-({[(9H-fluoren-9-yl)methoxy]carbonyl}(methyl)amino)hexanoic acid
(9H-Fluoren-9-yl)MethOxy]Carbonyl N-Me-Lys(Boc)-OH
Fmoc-N-Me-Lys(Boc)-OH
N-Fmoc-N'-Methyl-N'-Boc-Lysine
Fmoc-Nω-Me-Lys(Boc)-OH, >97%
FMOC-N-ME-LYS(BOC)-OH 1 G
FMOC-N-ME-LYS(BOC)-OH 250 MG
L-Lysine, N6-[(1,1-dimethylethoxy)carbonyl]-N2-[(9H-fluoren-9-ylmethoxy)carbonyl]-N2-methyl-
N-α-(9-Fluorenylmethoxycarbonyl)-N-α-methyl-N-ε-(t-butoxycarbonyl)-L-lysine
(2S)-2-[9H-fluoren-9-ylmethoxycarbonyl(methyl)amino]-6-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid
N6-Boc-N2-Fmoc-N2-methyl-L-lysine
FM
FM
197632-76-1
C27H34N2O6
amino acids





