ChemicalBook > Product Catalog >Chemical Reagents >Organic reagents >Esters >Ethyl (3-fluorobenzoyl)acetate
Ethyl (3-fluorobenzoyl)acetate
- CAS No.
- 33166-77-7
- Chemical Name:
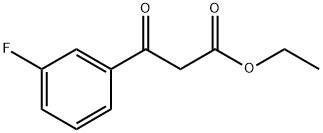
- Ethyl (3-fluorobenzoyl)acetate
- Synonyms
- Ethyl m-fluorobenzoyl acetate;Ethyl 2-3-fluorobenzoylacetate;ETHYL (3-FLUOROBENZOYL)ACETATE;Ethyl 3-fluoro-β-oxobenzenepropanoate;(m-Fluorobenzoyl)acetic acid ethyl ester;ETHYL 3-(3-FLUOROPHENYL)-3-OXOPROPANOATE;3-(3-Fluorophenyl)-3-oxopropanoic acid ethyl ester;Benzenepropanoic acid, 3-fluoro-β-oxo-, ethyl ester;3-(3-Fluoro-phenyl)-3-oxo-propionic acid ethyl bster;3-(3-FLUORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
- CBNumber:
- CB2148920
- Molecular Formula:
- C11H11FO3
- Molecular Weight:
- 210.2
- MOL File:
- 33166-77-7.mol
- Modify Date:
- 2025/1/27 9:38:02
| Boiling point | 262-263 °C(lit.) |
|---|---|
| Density | 1.166 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| form | Liquid |
| pka | 10.10±0.46(Predicted) |
| color | Colorless to orange |
| CAS DataBase Reference | 33166-77-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2916399090 | |||||||||
| NFPA 704 |
|
Ethyl (3-fluorobenzoyl)acetate price More Price(2)
Ethyl (3-fluorobenzoyl)acetate Chemical Properties,Uses,Production
Uses
Reactant for:
- Preparation of biologically active molecules
Synthesis
To a stirring suspension of NaH and CO(OEt)2 in toluene was added dropwise a solution of substituted acetophenone in toluene under reflux. The mixture was allowed to reflux and was stirred for 20 min after the addition was complete. When cooled to room temperature, the mixture was acidified with glacial HOAc. After ice-cold water was added, the mixture was extracted with toluene. The extract was then dried over anhydrous MgS04. After the toluene was evaporated in vacuo to give the corresponding substituted Ethyl (3-fluorobenzoyl)acetate.
Ethyl (3-fluorobenzoyl)acetate Preparation Products And Raw materials
Ethyl (3-fluorobenzoyl)acetate Suppliers
Global( 147)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | China | 8808 | 58 | Inquiry |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | China | 12810 | 58 | Inquiry |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | China | 10406 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49732 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29808 | 58 | Inquiry |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | China | 12162 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34563 | 58 | Inquiry |
| Shanghai Gubang New Materail Technology Co. LTD | 21-51317962 +8618721305404 | CHINA | 272 | 58 | Inquiry |
33166-77-7(Ethyl (3-fluorobenzoyl)acetate)Related Search:
Ethyl 1-ethyl-6,7,8-trifluoro-1,4-dihydro-4-oxoquinoline-3-carboxylate
3-(4-FLUORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
ETHYL 1,4-DIHYDRO-8-FLUORO-4-OXOQUINOLINE-3-CARBOXYLATE
3-(3,4-DIFLUOROPHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
Ethyl 2,4,5-trifluorobenzoylacetate
Ethyl 2,3,4,5-tetrafluorobenzoyl acetate
3-(2,4-DICHLORO-5-FLUORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
Ethyl (pentafluorobenzoyl)acetate
3-(2-FLUORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
5,8-DIFLUORO-4-OXO-1,4-DIHYDRO-QUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER
2-(2,4-DICHLORO-5-FLUORO-BENZOYL)-MALONIC ACID DIETHYL ESTER
Ethyl 6,7,8-trifluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate
(Z)-3-CYCLOPROPYLAMINO-2-(2,4-DICHLORO-5-FLUORO-BENZOYL)-ACRYLIC ACID ETHYL ESTER
7-CHLORO-1-CYCLOPROPYL-6-FLUORO-4-OXO-1,4-DIHYDRO-QUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER
2-CYANO-3-(2-FLUORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
chevron_left
1of4
chevron_right
3-(3-FLUORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
ETHYL 3-(3-FLUOROPHENYL)-3-OXOPROPANOATE
ETHYL (3-FLUOROBENZOYL)ACETATE
(m-Fluorobenzoyl)acetic acid ethyl ester
3-(3-Fluorophenyl)-3-oxopropanoic acid ethyl ester
Ethyl 2-3-fluorobenzoylacetate
Ethyl 3-fluoro-β-oxobenzenepropanoate
Ethyl m-fluorobenzoyl acetate
Benzenepropanoic acid, 3-fluoro-β-oxo-, ethyl ester
3-(3-Fluoro-phenyl)-3-oxo-propionic acid ethyl bster
33166-77-7
FC6H4COCH2CO2C2H5
Esters
Organic Building Blocks
C10 to C11
Carbonyl Compounds
Building Blocks
C10 to C11
Carbonyl Compounds
Esters





