ChemicalBook > Product Catalog >Inorganic chemistry >Inorganic salts >Metal halide and Halogen salt >Metal iodide and salt >MAGNESIUM IODATE
MAGNESIUM IODATE
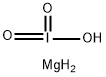
- CAS No.
- 7790-32-1
- Chemical Name:
- MAGNESIUM IODATE
- Synonyms
- MAGNESIUM IODATE;magnesium diiodate;MAGNESIUM IODATE 99.9%;MAGNESIUM IODATE 4-HYDRATE;Bisiodic acid magnesium salt;MAGNESIUM IODATE TETRAHYDRATE;Magnesium iodate tetrahydrate 99.0%;Magnesium iodate tetrahydrate, pure, 98%;MAGNESIUM IODATE TETRAHYDRATE, 98%, PURE;MAGNESIUM IODATE, TETRAHYDRATE PRIMARY STANDARD
- CBNumber:
- CB4328087
- Molecular Formula:
- H3IMgO3
- Molecular Weight:
- 202.23
- MOL File:
- 7790-32-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/17 17:11:47
| Melting point | 210 °C |
|---|---|
| Density | 3.300 |
| color | colorless monoclinic crystals, crystalline |
| Water Solubility | g/100g solution H2O: 8.55 (25°C), 13.5 (90°C); solid phase, Mg(IO3)2 · 4H2O (25°C), Mg(IO3)2 (90°C) [KRU93] |
| EPA Substance Registry System | Iodic acid (HIO3), magnesium salt (7790-32-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS07 |
|---|---|
| Signal word | Danger |
| Hazard statements | H272-H315-H319-H335 |
| Precautionary statements | P220-P261-P305+P351+P338 |
| Hazard Codes | O,Xi |
| Risk Statements | 8-36/37/38 |
| Safety Statements | 17-26-36 |
| RIDADR | UN 1479 5.1/PG 2 |
| WGK Germany | 3 |
MAGNESIUM IODATE Chemical Properties,Uses,Production
Chemical Properties
white crystalline chunks
Preparation
Magnesium Iodate can be prepared by reaction of iodic acid and magnesium
hydroxide:
Mg(OH)2+2HIO3→Mg(IO3)2+H2O
A tetrahydrate results that is relatively insoluble.
Magnesium iodate tetrahydrate occurs as welldefined
monoclinic crystals of high luster. It is easily
soluble in sulfuric acid.When heated, it loses two waters
of hydration at 510°C and the other two at 620°C.
Purification Methods
Crystallise it from water (0.2g/mL) between 100o and 0o.
MAGNESIUM IODATE Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 30)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 8853 | 58 | Inquiry |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | China | 2739 | 58 | Inquiry |
| JinJin Le Chemical Co., Ltd | 10106090 | China | 9981 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | China | 10011 | 58 | Inquiry |
| Barker Industries, Inc. | (704) 391-1023 | United States | 226 | 51 | Inquiry |
| Hangzhou Kaiyada Semiconductor Materials Co., LTD | 19817475709 | China | 474 | 58 | Inquiry |
| Sigma-Aldrich | 021-61415566 800-8193336 | China | 51471 | 80 | Inquiry |
| Jiangsu Aikon Biopharmaceutical R&D Co.,Ltd. | 025-66061636 18013972705 | China | 6763 | 58 | Inquiry |
| GFS Chemicals | 800 858-9682 (U.S. & Canada) | United States | 3404 | 75 | Inquiry |
7790-32-1(MAGNESIUM IODATE)Related Search:
MAGNESIUM IODATE 4-HYDRATE
MAGNESIUM IODATE TETRAHYDRATE
MAGNESIUM IODATE
magnesium diiodate
MAGNESIUM IODATE 99.9%
Magnesium iodate tetrahydrate, pure, 98%
MAGNESIUM IODATE, TETRAHYDRATE PRIMARY STANDARD
MAGNESIUM IODATE TETRAHYDRATE, 98%, PURE
Bisiodic acid magnesium salt
Magnesium iodate tetrahydrate 99.0%
7790-32-1
I2MgO64H2O
MgIO324H2O
Inorganic Salts
Synthetic Reagents
Inorganic Salts
Magnesium Salts
MagnesiumMetal and Ceramic Science
Salts
Synthetic Reagents





