SILVER BROMIDE
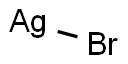
- CAS No.
- 7785-23-1
- Chemical Name:
- SILVER BROMIDE
- Synonyms
- AgBr;99.90%;bromosilver;SILVER BROMIDE;Silver brohide;SILVER(I) BROMIDE;Silver bromide,99%;Silver bromide 99%;silverbromide(agbr);SILVER BROMIDE: 99.9%
- CBNumber:
- CB5359545
- Molecular Formula:
- AgBr
- Molecular Weight:
- 187.77
- MOL File:
- 7785-23-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/4/3 16:52:12
| Melting point | 432 °C (lit.) |
|---|---|
| Boiling point | 700 °C |
| Density | 6.473 g/mL at 25 °C (lit.) |
| storage temp. | Room Temperature |
| solubility | insoluble in H2O, acid solutions, ethanol |
| form | Powder |
| Specific Gravity | 6.473 |
| color | Pale Yellow |
| Water Solubility | soluble in alkali cyanide solutions. Partially soluble in ammonia. Insoluble in alcohol and acids. Practically insoluble in water. |
| Sensitive | Light Sensitive |
| Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m |
| Merck | 14,8506 |
| Solubility Product Constant (Ksp) | pKsp: 12.3(25°C) |
| Dielectric constant | 12.2(0.0℃) |
| Stability | Stability Darkens in light |
| InChIKey | ADZWSOLPGZMUMY-UHFFFAOYSA-M |
| CAS DataBase Reference | 7785-23-1(CAS DataBase Reference) |
| EPA Substance Registry System | Silver bromide (AgBr) (7785-23-1) |
| Hardness, Mohs | 2.5 |
|---|
SILVER BROMIDE price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 226815 | Silver bromide 99% | 7785-23-1 | 5G | ₹2708.4 | 2022-06-14 | Buy |
| ALFA India | ALF-011425-22 | Silver bromide, 99.5% | 7785-23-1 | 100g | ₹45613 | 2022-05-26 | Buy |
| ALFA India | ALF-011425-14 | Silver bromide, 99.5% | 7785-23-1 | 25g | ₹17023 | 2022-05-26 | Buy |
| ALFA India | ALF-011425-06 | Silver bromide, 99.5% | 7785-23-1 | 5g | ₹3451 | 2022-05-26 | Buy |
| ottokemi | S 1501 | Silver bromide 98% | 7785-23-1 | 25gm | ₹7929 | 2022-05-26 | Buy |
SILVER BROMIDE Chemical Properties,Uses,Production
Chemical Properties
Silver bromide, AgBr, is pale yellow crystals or powder, that darken on exposure to light, finally turning black and soluble in potassium bromide, potassium cyanide, and sodium thiosulfate solutions, only very slightly soluble in ammonia water, insoluble in water, and light sensitive. Derivation is through silver nitrate dissolving in water and a solution ofalkali bromide added slowly. The precipitated silver bromide is washed repeatedly with hotwater. The operation must be carried on in a darkroom under a ruby-red light. Used in photographic film and plates, photochromic glass and as a laboratory reagent.
Physical properties
Yellow cubic crystals or powder; refractive index 2.253; darkens on exposure to light; Mohs hardness 2.5; density 6.47g/cm3; melts at 432°C; vaporizes at 1,502°C; insoluble in water, alcohol, and most acids; slightly soluble in dilute ammonia and ammonium carbonate solutions; sparingly soluble in concentrated ammonia solution (0.33 g/100mL 10% ammonia solution at 12°C);soluble in alkali cyanide solutions.
Uses
A yellowish powder made by the combination of any soluble bromide with silver nitrate. Silver bromide could also be formed by exposing metallic silver to the fumes of bromine as in the daguerreotype process. It is soluble in sodium thiosulfate, potassium bromide, and potassium cyanide solutions. Silver bromides were secondary halides for the paper negative processes, albumen negative process, and wet or preserved collodion processes. Silver bromides were the primary halides for the silver bromide collodion emulsion negative and the silver bromide gelatin emulsion processes. Silver bromide is the most photosensitive silver halide.
Preparation
Silver bromide is prepared by double decomposition reaction. An aqueous solution of alkali bromide, such as sodium or potassium bromide, is slowly added to an aqueous solution of silver nitrate:
Ag+ (aq) + Brˉ (aq) → AgBr(s)
The precipitate is washed repeatedly with hot water. Preparation should be in a dark room under a ruby red light.
Purification Methods
It is insoluble in dilute HNO3 or dilute NH3 but is soluble in conc NH3. Store it in the dark.
SILVER BROMIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| Micron Platers | +91-2229205235 +91-9821359130 | Maharashtra, India | 20 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3077 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Evans Fine Chem | 58 |
| JSK Chemicals | 58 |
| Dhara Industries | 58 |
| Micron Platers | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Triveni chemicals | 58 |
| LOBA CHEMIE PVT.LTD. | 58 |
| Central Drug House(P) Ltd. | 58 |
Related articles
- Crystal Structure and Synthesis of Silver Bromide Complex
- This article will introduce the crystal structure and synthesis method of a silver bromide complex.
- Apr 3,2024
- How Is Silver Bromide Used in Photography?
- Silver bromide is used in photography as a component of an emulsion that helps develop a photographic image. Silver bromide is....
- Nov 6,2019
7785-23-1(SILVER BROMIDE)Related Search:
1of4
chevron_right





