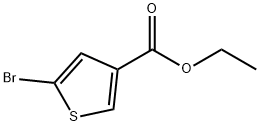
Ethyl 5-bromothiophene-3-carboxylate
- CAS No.
- 170355-38-1
- Chemical Name:
- Ethyl 5-bromothiophene-3-carboxylate
- Synonyms
- Ethyl 5-bromothiophene-4-carboxylate;Ethyl 5-bromothiophene-3-carboxylate;Ethyl 5-bromothiophene-3-carboxylate 97+%;5-bromo-3-Thiophenecarboxylic acid ethyl...;PI-35048 ethyl 5-bromothiophene-3-carboxylate;5-bromo-3-Thiophenecarboxylic acid ethyl ester;5-BroMo-thiophene-3-carboxylic acid ethyl ester;3-Thiophenecarboxylic acid, 5-bromo-, ethyl ester;5-Bromothiophene-3-carboxylic acid ethyl ester, >=98%;5-bromo-3-Thiophenecarboxylic acid ethyl ester (9CI ACI)
- CBNumber:
- CB62514598
- Molecular Formula:
- C7H7BrO2S
- Molecular Weight:
- 235.1
- MOL File:
- 170355-38-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/7 10:46:57
| Boiling point | 65-66 °C |
|---|---|
| Density | 1.572±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| form | liquid |
| color | Colourless to yellow |
| InChI | InChI=1S/C7H7BrO2S/c1-2-10-7(9)5-3-6(8)11-4-5/h3-4H,2H2,1H3 |
| InChIKey | LUYMKCLOYODOEI-UHFFFAOYSA-N |
| SMILES | C1SC(Br)=CC=1C(OCC)=O |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319 |
| Precautionary statements | P501-P270-P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313-P301+P312+P330 |
| HS Code | 2934999090 |
Ethyl 5-bromothiophene-3-carboxylate Chemical Properties,Uses,Production
Synthesis
20 g (147 mmol) of aluminum chloride are added portion-wise to a solution of 10 g (67 mmol) of ethyl thiophene-3-carboxylate in 160 ml of dichloro-methane, cooled to 0oC. The reaction medium is warmed to room temperature, and a solution of 4 ml (73 mmol) of bromine in 10 ml of dichloromethane is added. After reaction for 50 minutes at room temperature, the reaction medium is poured into a water + ice mixture and extracted with dichloromethane. The organic phase is dried over magnesium sulfate, filtered, and evaporated under vacuum. The residue obtained is purified by chromatography on a column of silica eluted with a 9/1 HEPTANE/ETHYL acetate mixture. 9 g (57percent) of ethyl 5-bromothiophene-3-carboxylate are obtained.
Uses
Ethyl 5-bromothiophene-3-carboxylate is an important organic reagent that can be used as a building block for the synthesis of many organic compounds. After hydrolysis, it can synthesize 5-Bromothiophene-3-carboxylic acid.
Ethyl 5-bromothiophene-3-carboxylate Preparation Products And Raw materials
Raw materials
Preparation Products
Ethyl 5-bromothiophene-3-carboxylate Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Shanxi Tihondan Pharmaceutical Technology Co., Ltd | +8618235132063 | China | 3725 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | China | 9624 | 58 | Inquiry |
| Huida Pharmaceutical Technology (Shanghai) Co., Ltd. | +8613601750004 | China | 439 | 58 | Inquiry |
| Henan Allgreen Chemical Co.,LTD | +86-37155567971 +86-13633837469 | China | 5986 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20308 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Nanjing Bicbiotechnology Co., Ltd | +86-2552131256 +86-18251840740 | China | 6000 | 58 | Inquiry |





