Umbralisib
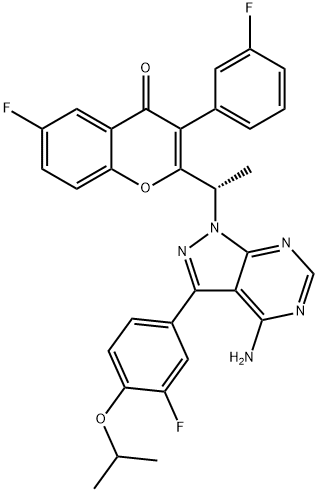
- CAS No.
- 1532533-67-7
- Chemical Name:
- Umbralisib
- Synonyms
- CS-2443;TGR1205;RP-5264;TGR-1202;Umbralisib;RP-5264 TGR-1202;RP5264;UMBRALISIB;TGR-1202(Umbralisib);Umbralisib (TGR-1202);RP5264; TGR1202; TGR 1202; RP-5264; RP 5264
- CBNumber:
- CB62716383
- Molecular Formula:
- C31H24F3N5O3
- Molecular Weight:
- 571.55
- MOL File:
- 1532533-67-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:13
Umbralisib Chemical Properties,Uses,Production
Description
Umbralisib (TGR-1202) tosylate is an orally active, potent and selective dual PI3Kδ and casein kinase-1-ε (CK1ε) inhibitor, with EC50 of 22.2 nM and 6.0 μM, respectively. Umbralisib tosylate exhibits unique immunomodulatory effects on chronic lymphocytic leukemia (CLL) T cells. Umbralisib tosylate can be used for haematological malignancies reseach.
Uses
RP 5264 is a novel PI3K inhibitor that enhances Brentuximab Vedotin-induced lymphoma cell death.
brand name
Ukonig
General Description
Class: lipid kinase; Treatment: MZL, FL; Other name: TGR-1202; Elimination half-life = 91 h; Protein binding > 99.7%
Pharmacokinetics
Umbralisib has a prolonged half-life of 91 h, which enables once-daily dosing. However, the recommended dosage of 800 mg is the highest among all the PI3K inhibitors in use, presumably due to its limited oral bioavailability.
Synthesis
The synthesis of Umbralisib is as follows:
To a solution of intermediate 13 (0.134 g, 0.494 mmol) in THF (2.0 ml), intermediate 5 (0.150 g, 0.494 mmol) and triphenylphosphine (0.194 g, 0.741 mml) were added and stirred at RT for 5 min. Diisopropylazodicarboxylate ( 0.15 ml, 0.749 mmol) was added heated to 45°C. After 2h, the reaction mixture was quenched with with water and extracted with ethyl acetate. The organic layer was dried over sodium sulphate and concentrated under reduced pressure. The crude product was purified by column chromatography with ethyl acetate : petroleum ether to afford Umbralisib as an off-white solid (0.049 g, 20 %).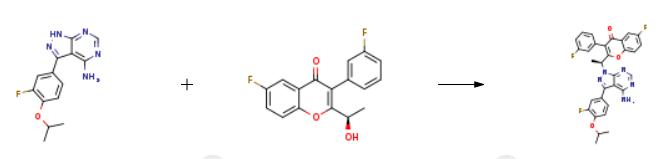
target
PI3Kδ, CK1ε
Umbralisib Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
Umbralisib Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Wuhan Demeikai Biotechnology Co., Ltd | +8618942921723 | China | 723 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
| Nanjing Doge Biomedical Technology Co., Ltd | +86-25-58227606 +86-15305155328 | China | 4128 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 | United States | 19973 | 58 | Inquiry |
| Shanghai Huici Pharmaceutical Technology Co., LTD | +8618917134367 | China | 133 | 58 | Inquiry |
| Shanghai Huici Pharmaceutical Technology Co., LTD | +8618917134367 | China | 133 | 58 | Inquiry |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | China | 8474 | 58 | Inquiry |
| LEAPCHEM CO., LTD. | +86-852-30606658 | China | 43348 | 58 | Inquiry |







